Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Breast Disorders
Breast Cancer
Breast Cancer
There
is no single, specific cause of breast cancer; rather, a com-bination of
hormonal, genetic, and possibly environmental events may contribute to its
development.
Etiology
Hormones
produced by the ovaries have an important role in breast cancer. Two key
ovarian hormones, estradiol and proges-terone, are altered in the cellular
environment by a variety of fac-tors, and these may affect growth factors for
breast cancer.
HORMONES
The
role of hormones and their relationship to breast cancer re-main controversial.
Research suggests that a relationship exists between estrogen exposure and the
development of breast can-cer. In laboratory studies, tumors grow much faster
when ex-posed to estrogen, and epidemiologic research suggests that women who
have longer exposure to estrogen have a higher risk for breast cancer. Early
menarche, nulliparity, childbirth after 30 years of age, and late menopause are
known but minor risk factors. The assumption is that these factors are all
associated with prolonged exposure to estrogen because of menstruation. The
theory is that each cycle (which has high levels of endoge-nous estrogen)
provides the cells of the breast another chance to mutate, increasing the
chance for cancer to develop. Estrogen itself does not cause breast cancer, but
it is associated with its development.
GENETICS
Growing
evidence indicates that genetic alterations are associated with the development
of breast cancer. These genetic alterations include changes or mutations in
normal genes and the influence of proteins that either promote or suppress the
development of breast cancer. Genetic alterations may be somatic (acquired) or
germline (inherited). To date, two gene mutations have been iden-tified that
may play a role in the development of breast cancer. A mutation in the BRCA-1 gene has been linked to the
develop-ment of breast and ovarian cancer, whereas a mutation in the BRCA-2 gene identifies risk for breast
cancer, but less so for ovar-ian cancer (Houshmand, Campbell, Briggs et al.,
2000). These gene mutations may also play a role in the development of colon,
prostate, and pancreatic cancer, but this is far from clear at pres-ent. It has
been estimated that 1 of 600 women in the general population has either a
BRCA-1 or BRCA-2 gene mutation. For women who carry either mutation, the risk
for developing breast cancer can range from 50% to 90% (Kauff, Satagopan,
Robson et al., 2002).
At
present, only 5% to 10% of all breast cancers are estimated to be associated
with the BRCA-1 or BRCA-2 gene mutations. It is thought, however, that breast
cancer is genetic and that up to 80% of women diagnosed with breast cancer
before age 50 years have a genetic component to their disease (Boyd, 1996).
This is believed to be linked to either unidentified BRCA-1 or BRCA-2 carriers
or less penetrating genes that have yet to be identified through genetics
research. A woman’s risk for either BRCA-1 or BRCA-2 should be interpreted with
caution and with an exhaus-tive look at all her other risk factors; this is
usually carried out by a genetics counselor.
Abnormalities
in either of the two genes can be identified by a blood test; however, women
should be counseled about the risks and benefits before actually undergoing
genetic testing. The risks and benefits of a positive or negative result should
be explored. Treatment options for a positive result are long-term
surveillance, bilateral prophylactic mastectomy, or chemoprevention with
ta-moxifen, as discussed previously. A positive result can cause tremendous
anxiety and fear, can unleash potential discrimina-tion in employment and
insurability, and can cause a woman to search for answers that may not be
available. A negative result can produce survivor guilt in a person with a
strong family history of cancer. For these women, the risk for breast cancer is
similar to that of the general population, and routine screening guidelines
should be followed. The decision to pursue genetic testing must be made
carefully, and women should be asked what they will do differently after they
know the results. Furthermore, because testing is relatively new and health
care providers have yet to de-termine a true benefit from a positive or
negative result, genetic testing should be done under the auspices of clinical
research pro-tocols to protect the patient (because these data are kept
separate from the patient’s medical record). Nurses play a role in educat-ing
patients and their family members about the implications of genetic testing.
Ethical issues related to genetic testing include possible employment
discrimination, bias in insurability and pos-sibly with insurance rates, and
family members’ concerns (eg, effect on siblings, children).
Risk Factors
Although
there are no specific known causes of breast cancer, re-searchers have
identified a cluster of risk factors (Chart 48-3). These factors are important
in helping to develop prevention pro-grams. However, nearly 60% of women
diagnosed with breast cancer have no identifiable risk factors other than their
hormonal environment (Vogel, 2000). Thus, all women are considered at risk for
developing breast cancer during their lifetime. Nonethe-less, identifying risk
factors provides a means for identifying women who may benefit from increased
surveillance and early treatment. In addition, further research into risk
factors will help in developing strategies to prevent or modify breast cancer
in the future.

A
high-fat diet was once thought to increase the risk of breast cancer.
Epidemiologic studies of American and Japanese women showed that American women
had a fivefold higher rate of breast cancer. Japanese women who moved to the
United States were shown to have breast cancer rates similar to their Caucasian
counterparts. Recent cohort studies show only weak or incon-clusive relationships
between a high-fat diet and breast cancer (Brown et al., 2001). Because fat
intake is implicated in colon cancer and heart disease, however, women may
benefit from lowering their intake of fat.
Oral
contraceptives were once thought to increase the risk for breast cancer.
Currently, no association is thought to exist in women in the general
population, but there are no data about the effect on women considered to be at
high risk.
The
role of smoking in breast cancer remains unclear. Most studies suggest that
smoking does not increase a woman’s risk for breast cancer. Some studies,
however, suggest that smoking does increase the risk for breast cancer and that
the earlier a woman be-gins smoking, the higher her risk. Smoking does increase
the risk for lung cancer, which is the leading cause of death in women with
cancer (breast cancer is second). Smoking cessation is part of a healthy
lifestyle, and nurses have a key role in providing women with information about
smoking cessation programs.
Silicone
breast implants can be associated with fibrous capsu-lar contraction, and some
women and medical professionals have claimed an association with certain immune
disorders. There is no evidence, however, that breast implants are associated
with an increased risk of breast cancer.
Protective Factors
Certain factors may be protective in relation to the development of breast cancer. Regular, vigorous exercise has been shown to de-crease risk, perhaps because it can delay menarche, suppress men-struation, and, like pregnancy, reduce the number of ovulatory menstrual cycles. Also, exercise decreases body fat, where estro-gens are stored and produced from other steroid hormones. Thus, decreased body fat can decrease extended exposure to estrogen.
Breastfeeding
is also thought to decrease risk because it prevents the return of
menstruation, again decreasing exposure to endo-genous estrogen. Having had a
full-term pregnancy before the age of 30 years is also thought to be
protective. Protective hormones are released after delivery of the fetus, with
the purpose of revert-ing to normal the proliferation of cells in the breast
that occur with pregnancy.
Clinical Manifestations
Breast
cancers occur anywhere in the breast, but most are found in the upper outer
quadrant, where most breast tissue is located. Generally, the lesions are
nontender rather than painful, fixed rather than mobile, and hard with
irregular borders rather than encapsulated and smooth. Complaints of diffuse
breast pain and tenderness with menstruation are usually associated with benign
breast disease. Marked pain at presentation, however, may be associated with
breast cancer in the later stages.
With
the increased use of mammography, more women are seeking treatment at an earlier
stage of disease. These women may have no symptoms and no palpable lump, but
abnormal lesions are detected on mammography. Unfortunately, many women with
advanced disease seek initial treatment only after ignoring symptoms. For
example, they may seek attention for dimpling or for a peau d’orange
(orange-peel) appearance of the skin (a condi-tion caused by swelling that
results from obstructed lymphatic cir-culation in the dermal layer). Nipple
retraction and lesions fixed to the chest wall may also be evident. Involvement
of the skin is manifested by ulcerating and fungating lesions. These classic
signs and symptoms characterize breast cancer in the late stages. A high index
of suspicion should be maintained with any breast abnor-mality, and abnormalities
should be promptly evaluated.
Assessment and Diagnostic Findings
Techniques
to determine the histology and tissue diagnosis of breast cancer include FNA,
excisional (or open) biopsy, incisional biopsy, needle localization, core
biopsy, and stereotactic biopsy (all described previously). In addition to the
staging criteria de-scribed below, other pathologic features and prognostic
tests are used to identify different patient groups that may benefit from
adjuvant treatment. Histologic examination of the cancer cells helps determine
the prognosis and leads to a better understand-ing of how the disease
progresses.
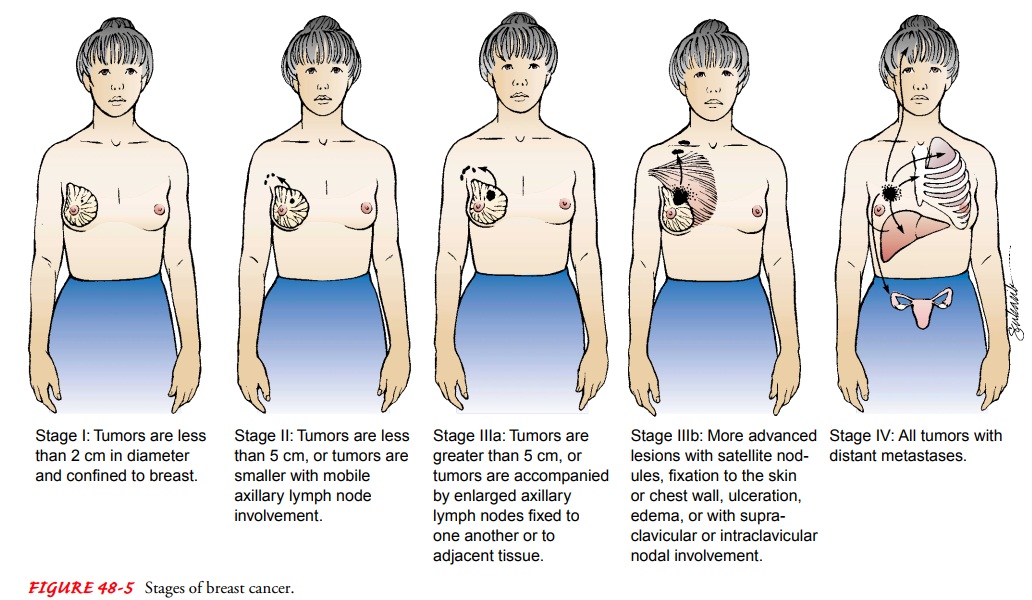
Breast Cancer Staging
Staging
involves classifying the cancer by the extent of disease (see Fig. 48-5).
Staging of any cancer is important because it helps the health care team
identify and recommend the best treatment available, offer a prognosis, and
compare the results of various treatment regimens. Several diagnostic tests and
procedures are performed in the staging of the disease. These may include chest
x-rays, bone scans, and liver function tests. Clinical staging in-volves the
physician’s estimate of the size of the breast tumor and the extent of axillary
node involvement by physical examination (palpable nodes may indicate
progression of the disease) and mammography. After the diagnostic workup and
the definitive surgical treatment, the breast cancer is staged according to the
TNM system (Greene, Page, Fleming, et al., 2002), which evaluates the size of
the tumor, number of nodes involved, and evidence of dis-tant metastasis.
Pathologic staging based on histology provides information for a more accurate
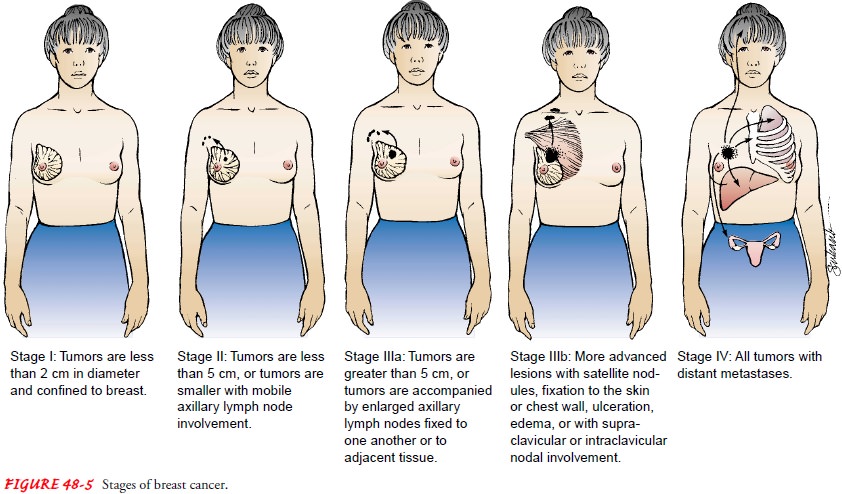
prognosis. Table 48-2 lists typi-cal treatment guidelines by stage at
diagnosis.
Prognosis
Several features of breast tumors contribute to the prognosis. Generally, the smaller the tumor, the better the prognosis. Car-cinoma of the breast is not a pathologic entity that develops overnight. It starts with a genetic alteration in a single cell. It can take about 16 doubling times for a carcinoma to become 1 cm or larger, at which point it becomes clinically apparent. Assuming that it takes at least 30 days for each doubling time, it would take a minimum of 2 years for a carcinoma to become palpable. This concept is important for nurses in teaching and counseling pa-tients because once breast cancer is diagnosed, women have a safe period of several weeks to make a decision regarding treatment.
The
prognosis also depends on whether the cancer has spread. For example, the
overall 5-year survival rate is greater than 98% when the tumor is confined to
the breast (ACS, 2002). When the cancer cells have spread to the regional lymph
nodes, however, the overall 5-year survival rate falls to 76%. The 5-year
survival rate for women diagnosed with metastatic disease is 16%. At
diagno-sis, about 37% of patients have evidence of regional or distant spread
or metastasis. The most common route of regional spread is to the axillary
lymph nodes. Table 48-3 describes the relation-ship between positive axillary
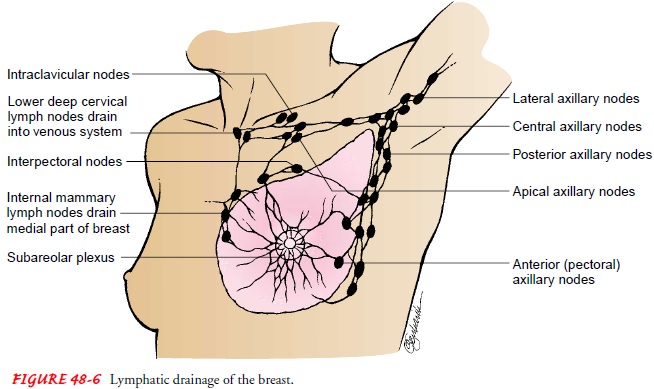
lymph nodes and the risk for breast cancer recurrence. Other sites of lymphatic
spread include the in-ternal mammary and supraclavicular nodes (Fig. 48-6).
Distant metastasis can affect any organ, but the most common sites are bone
(71%), lung (69%), liver (65%), pleura (51%), adrenals (49%), skin (30%), and
brain (20%) (Winchester & Cox, 1998).

In addition to tumor size, nodal involvement, evidence of metastasis, and histologic type, other measures help in determin-ing prognosis. The presence of estrogen and progesterone recep-tor proteins indicates retention of regulatory controls of the mammary epithelium. The presence of both receptor proteins is associated with an improved prognosis; their absence is associated with a poorer prognosis. Similarly, a tumor with a high degree of differentiation is associated with a better prognosis than a poorly differentiated anaplastic tumor. The assessment of a tumor’s pro-liferative rate (S-phase fraction) and DNA content (ploidy) by lab-oratory assay may help to determine prognosis because these two factors are strongly correlated with other prognostic factors, and research is ongoing to examine how helpful these two factors may actually be. Tumors classified as diploid (normal DNA content) are associated with a better prognosis than are tumors classified as aneuploid (abnormal DNA content).
Related Topics