Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Breast Disorders
Breast Cancer: Medical Management
Medical
Management
CHANGING APPROACHES
In
1990, the National Institutes of Health Consensus Development Conference on
Breast Cancer issued its third statement on the management of breast cancer.
Based on worldwide data, breast-conserving surgery (such as lumpectomy), along
with radiation therapy, was found to result in a survival rate equal to that of
modified radical mastectomy. In addition, recommendations were made for
systemic treatment with chemotherapy based on the patient’s menopausal status
and the presence of hormone re-ceptors. For a premenopausal woman without involvement
of the lymph nodes, adjuvant chemotherapy was recommended if the woman was at
high risk for recurrence. For a postmenopausal woman without involvement of the
lymph nodes, adjuvant chemo-therapy was not recommended regardless of hormonal
receptor status. For a premenopausal woman with involvement of the nodes,
adjuvant chemotherapy was recommended. In a postmenopausal woman, hormone
therapy was recommended if the woman had an estrogen receptor-positive tumor.
In
1991, the National Cancer Institute issued a clinical alert that altered the
recommendations of the 1990 Consensus Devel-opment Conference Statement. This
alert recommended that all premenopausal, node-negative women at high risk for
recurrent disease receive adjuvant chemotherapy. This clinical alert was
is-sued before the results of clinical trials were published, creating
confusion among clinicians and patients alike. The 2000 Con-sensus Development
Conference Statement stated that all women with invasive breast cancer should
consider systemic chemother-apy, not just women with tumors larger than 1 cm
(NIH, 2000).
Decisions
regarding local treatment with either mastectomy or breast-conserving surgery
with radiation still vary widely. Mas-tectomy is still performed in many cases,
and rates for breast-conserving surgery are higher in metropolitan areas with
teaching and research hospitals and medical centers. In the past, women have
not routinely been presented with the option of breast-conserving surgery by
their physicians, and in many instances in-surance reimbursement patterns favor
mastectomy. Thus, women have not uniformly had the opportunity to exercise
informed choice in their options for local treatment, but this is changing as
women become more knowledgeable about breast cancer and its treatment. A second
opinion regarding treatment options is usu-ally helpful to women diagnosed with
breast cancer.
SURGICAL MANAGEMENT
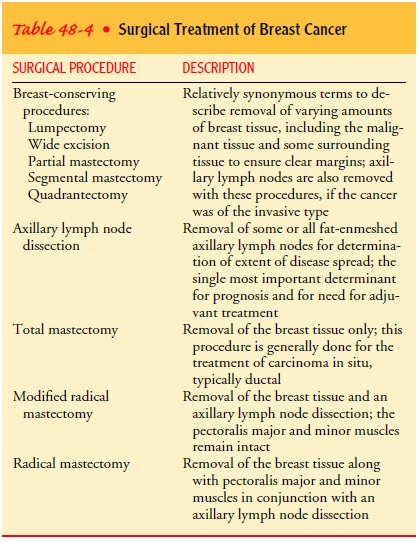
The main goal of surgical treatment is to eradicate the local pres-ence of the cancer. The procedures most often used for the local management of invasive breast cancer are mastectomy with or without reconstruction and breast-conserving surgery combined with radiation therapy. These procedures are described below. Surgical treatment options are summarized in Table 48-4. For pa-tients who undergo total mastectomy for the treatment of DCIS or as prophylactic surgery for the treatment for LCIS, the nursing care is similar to that of a modified radical mastectomy (described later in the text).
However, total mastectomy does not involve the removal
of axillary lymph nodes; therefore, mobility of the arm on the affected side is
regained much quicker, and there is no risk for lymphedema. Women still face
the same psychosocial issues involving the diagnosis of cancer and the loss of
the breast, and the nurse needs to address these in a similar manner.
Modified Radical Mastectomy.
Modified radical mastectomyisremoval of
the entire breast tissue, along with axillary lymph nodes. The pectoralis major
and pectoralis minor muscles remain intact. Before surgery, the surgeon plans
an incision that will pro-vide maximum opportunity to remove the tumor and the
affected nodes. At the same time, efforts are made to avoid a scar that will be
visible and restrictive. An objective of surgical treatment is to maintain or
restore normal function to the hand, arm, and shoul-der girdle on the affected
side. Skin flaps and tissue are handled with great care to ensure proper
viability, hemostasis, and drain-age. If reconstructive surgery is planned, a
consultation is made with a plastic surgeon before the mastectomy is performed.
After
the tumor is removed, bleeding points are ligated and the skin is closed over
the chest wall. Skin grafting is performed if the skin flaps are too small to
close the wound. A nonadherent dressing (Adaptic) may be applied and covered by
a pressure dress-ing. Two drainage tubes may be placed in the axilla and
beneath the superior skin flap, and portable suction devices may be used; these
remove the blood and lymph fluid that collect after surgery. The dressing may
be held in place by wide elastic bandages or a surgical bra.
Breast-Conserving Surgery.
Breast-conserving
surgery consistsof lumpectomy, wide excision, partial or segmental mastectomy,
or quadrantectomy (resection of the involved breast quadrant) and removal of
the axillary nodes (axillary lymph node dissection) for tumors with an invasive
component, followed by a course of radiation therapy to treat residual,
microscopic disease.
The
goal of breast conservation is to remove the tumor com-pletely with clear
margins while achieving an acceptable cosmetic result. The axillary lymph nodes
are also removed through a sep-arate semicircular incision under the hair-bearing
portion of the axilla. A drain is inserted into the axilla through a separate
stab wound to remove blood and lymph fluid. A dressing is applied over the
breast and under the arm and is secured with wide elas-tic bandages or a
surgical bra.
Survival
rates after breast-conserving surgery are equivalent to those after modified
radical mastectomy. The risk for local recur-rence, however, is greater, at 1%
per year after surgery (Winchester Cox, 1998). If the patient experiences a
local recurrence, stan-dard treatment is a completion or salvage mastectomy, in
which the rest of the breast tissue is removed. Survival rates after this
proce-dure are equivalent to those after mastectomy, but because the skin has
been irradiated, the choices for reconstruction remain lim-ited, and the woman
should be informed of this possibility at the time of diagnosis and when
considering her treatment options.
Postoperative Issues.
The
postoperative care of the patient un-dergoing a modified radical mastectomy or
breast-conserving surgery is similar because both procedures involve an
alteration to the breast and removal of lymph nodes from the axillary re-gion.
As with any surgical patient, the immediate focus is recov-ery from general
anesthesia and pain management. In addition, the patient who has had breast
surgery may experience both phys-ical and psychological effects. Possible
complications include the accumulation of blood (hematoma) at the incision
site, infection, and late accumulation of serosanguineous fluid (seroma) after
drain removal. Most patients who undergo breast surgery are dis-charged home
with a drainage collection device in place. The nurse teaches the patient and
family members to manage the drainage system.
Nerve
trauma with resultant phantom breast sensations, numb-ness, tingling, or
burning sensations may also occur and may per-sist for months or possibly years
after surgery. Impaired arm and shoulder mobility can result from the axillary
dissection. The dis-ruption of lymphatic and venous drainage can leave the
patient at risk for lymphedema
(chronic swelling of the affected extremity) at any point after surgery.
Psy-chological sequelae may include an altered body image or self-concept as a
result of the alteration or loss of the breast. Other major psychosocial
concerns include uncertainty about the fu-ture, fear of recurrence, and the
effects of breast cancer and its treatment on family and work roles.
Lymphatic Mapping and Sentinel Node Biopsy.
In the mid 1990s,a new procedure was introduced for use in patients
undergoing breast surgery for the treatment of invasive breast cancer.
Approx-imately 60% of patients who undergo an axillary lymph node dis-section
to determine the extent of the disease have negative nodes (Veronesi,
Galimberti, Zurrida et al., 2001). The use of lym-phatic mapping and sentinel node biopsy is changing the
waythese patients are treated because it provides the same prognostic
information as the axillary dissection. A radiocolloid and/or blue dye is
injected into the tumor site; the patient then undergoes the surgical
procedure. The surgeon uses a hand-held probe to locate the sentinel node (the
primary drainage site from the breast) and excises it, and it is examined by
the pathologist. If the sen-tinel node is negative for metastatic breast
cancer, a standard axil-lary dissection is not needed, thus sparing the patient
the sequelae of the procedure (surgical drain, altered mobility of the
extrem-ity, paresthesias, risk for lymphedema). If the sentinel node is
positive, the patient undergoes the standard axillary dissection. Reported
results of this technique suggest a success rate of more than 90% in correctly
identifying the sentinel node and correctly predicting axillary metastases
(Hsueh, Hansen & Guiliano, 2000), and many centers have incorporated this
procedure into standards
of care. Short-term follow-up demonstrates that the rate of lymphedema is
approximately 1% for women who undergo sentinel lymph node biopsy.
Nursing
issues for this procedure focus on informing the pa-tient about the
expectations and possible implications. Because patients with a negative node
are spared the axillary dissection, they may be discharged home the same day.
Research is needed on the technique’s sequelae, however. Questions to be
addressed in-clude: Do patients experience similar sensations in the affected
arm as those who had an axillary dissection? Do patients demon-strate impaired
mobility? Do these patients develop axillary sero-mas after the procedure? What
is the risk for lymphedema? Initial nursing research on patient care issues
related to sentinel node biopsy demonstrates that women who have sentinel node
biopsy alone do have neuropathic sensations similar to those who undergo an
axillary dissection, although the prevalence, severity, and dis-tress are less
so (Baron, Fey, Raboy et al., 2002).
RADIATION THERAPY
With
breast-conserving surgery, a course of external-beam radia-tion therapy usually
follows excision of the tumor mass to de-crease the chance of local recurrence
and to eradicate any residual microscopic cancer cells. Radiation treatment is
necessary to ob-tain results equal to those of removal of the breast. If
radiation therapy is contraindicated, mastectomy is the patient’s only option
(Chart 48-4).
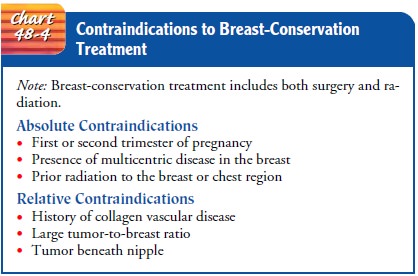
Radiation
treatment typically begins about 6 weeks after the surgery to allow the
incision to heal. If systemic chemotherapy is indicated, radiation therapy
usually begins after completion of the chemotherapy. External-beam irradiation
provided by a lin-ear accelerator using photons is delivered on a daily basis
over 5 to 7 weeks to the entire breast region. In addition, a concentrated
radiation dose or “boost” is administered to the primary site by means of
electrons. Before radiation therapy begins, the patient undergoes a planning
session for radiation treatment that will serve as the model for daily
treatments. Small permanent ink mark-ings are used to identify the breast
tissue to be irradiated. Patients need reassurance about the procedure and
self-care instructions related to side effects and their management.
Postoperative radiation after mastectomy is not common today but is still used in certain cases: when tumors have spread re-gionally (chest wall involvement, four or more positive nodes, or tumors larger than 5 cm). Occasionally, patients who have had a mastectomy require radiation treatment to the chest wall, gener-ally after completion of systemic chemotherapy. Treatment usu-ally consists of a course of external-beam irradiation to the area for a period of several weeks, but the time frame is determined by the radiation oncologist. Some studies suggest that survival may be enhanced for high-risk premenopausal women who receive chest wall irradiation after mastectomy.
Another
approach to radiation therapy is the use of intraoper-ative radiation therapy
(IORT), in which a single dose of radia-tion is delivered to the lumpectomy
site immediately after the surgeon has performed the lumpectomy. The dosage is
limited to the tumor area, as any errant cells are most likely to be within the
approximate area. The typical side effects of skin changes and fa-tigue are
minimized with this approach. It may be as effective as the standard 5- to
7-week radiation course, but long-term data are still needed to determine the
effect on local recurrence and survival rates.
Postirradiation Reaction.
Generally,
radiation therapy is welltolerated. Side effects are temporary and usually
consist of mild to moderate skin reaction and fatigue. Fatigue usually occurs
about 2 weeks after treatment and may last for several weeks after the
treatments are completed. Fatigue can be depressing, as can the frequent trips
to the radiation oncology unit or department for treatment. The patient needs
to be reassured that the fatigue is normal and not a sign of recurrence. Rare
complications of ra-diation therapy to the breast include pneumonitis, rib
fracture, and breast fibrosis.
Postirradiation Nursing Management.
Self-care
instructions forpatients receiving radiation are based on maintaining skin
in-tegrity during and after radiation therapy:
· Use mild soap with
minimal rubbing.
· Avoid perfumed soaps or
deodorants.
· Use hydrophilic lotions
(Lubriderm, Eucerin, Aquaphor) for dryness.
· Use a nondrying,
antipruritic soap (Aveeno) if itching occurs.
· Avoid tight clothes,
underwire bras, excessive temperatures, and ultraviolet light.
Patients
may note increased redness and, rarely, skin break-down at the booster site
(tissue site that received concentrated ra-diation). Important aspects of
follow-up care include teaching patients to minimize exposure of the treated
area to the sun for 1 year and reassurance that minor twinges and shooting pain
in the breast are normal reactions after radiation treatment.
CHEMOTHERAPY
Chemotherapy
is administered to eradicate the micrometastatic spread of the disease.
Although chemotherapy is generally initiated after breast surgery, no single
standard exists for the sequencing of sys-temic chemotherapy and radiation
therapy. Ongoing clinical tri-als may help to determine which treatment
sequence produces the best outcomes.
Chemotherapy
regimens for breast cancer combine several agents to increase tumor cell
destruction and to minimize med-ication resistance. The chemotherapeutic agents
most often used in combination are cyclophosphamide (Cytoxan) (C),
metho-trexate (M), fluorouracil (F), and doxorubicin (Adriamycin) (A).
Paclitaxel (Taxol) (T) has been recently introduced into the ad-juvant
chemotherapy setting, and the data from clinical trials suggest a slight
survival benefit with its use (Norton, 2001). Addi-tionally, a newer taxane,
docetaxel (Taxotere) (T), is being used more frequently, but research remains
limited on its difference. The combination regimen of CMF or CAF is a common
treatment protocol. AC, ACT (AC given first followed by T), and ATC, with all
three agents given together, are other regimens that may be used (Levine,
2001). A new anthracycline agent, epiru-bicin (Ellence), which has been used
more in Europe, is being used in certain regimens and protocols. Decisions
regarding the chemotherapeutic protocol are based on the patient’s age,
physi-cal status, and disease status and whether she is participating in a
clinical trial. Chemotherapy treatment modalities are summa-rized in Table
48-5.

Reactions to Chemotherapy.
Anticipatory
anxiety is a commonresponse among patients facing chemotherapy. Today, however,
side effects can be managed well, with many women continuing their daily work
and routine schedules. This has occurred in large measure because of the
meticulous educational and psychological preparation provided to patients and
their families by oncology nurses, oncologists, social workers, and other
members of the health care team. The other factor is the availability of medication
regimens that can alleviate the side effects of nausea and vomiting.
Common
physical side effects of chemotherapy for breast can-cer include nausea,
vomiting, taste changes, alopecia (hair loss), mucositis, dermatitis, fatigue,
weight gain, and bone marrow suppression. In addition, premenopausal women may
experience temporary or permanent amenorrhea leading to sterility.
Less
common side effects include hemorrhagic cystitis and conjunctivitis. Although
its cause is unknown, weight gain of more than 10 pounds occurs in about half
of all patients. Aero-bic exercise and its anxiety-alleviating effects may be
helpful to decrease weight gain and elevate mood. Side effects may vary with
the chemotherapeutic agent used. CMF is generally well tolerated with only
minimal side effects. Doxorubicin can be toxic to tis-sue if it infiltrates the
vein, so it is usually diluted and infused through a large vein. Nausea and
vomiting can occur. Antiemet-ics and tranquilizers may provide relief, as may
visual imagery and relaxation exercises. Doxorubicin and paclitaxel usually
cause alopecia, so obtaining a wig before hair loss occurs may prevent some of
the associated emotional trauma. The patient needs re-assurance that new hair
will grow when treatment is completed, although the color and texture of the
hair may differ. It is help-ful to provide a list of wig suppliers in the
patient’s geographic region and to become familiar with creative ways to use
scarves and turbans to reduce the patient’s reactions to hair loss. The
American Cancer Society offers a program called “Look Good, Feel Better” that
provides useful tips for applying cosmetics during chemotherapy.
Nursing Management in Chemotherapy.
Nurses
working withpatients receiving chemotherapy play an important role in
assist-ing those who have difficulty with the side effects of treatment.
Encouraging the use of medications to limit nausea, vomiting, and mouth sores
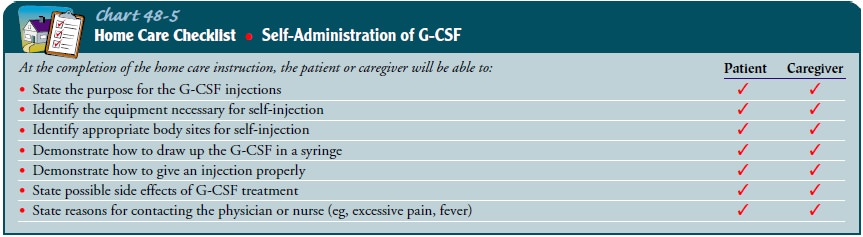
reduces discomfort during chemotherapy. Some pa-tients may receive granulocyte
colony-stimulating factor (G-CSF), synthetic growth-stimulating factor injected
subcutaneously daily for 10 days, which boosts the white blood cell count to
pre-vent nadir fever (a fever that occurs with infection when the blood cell
counts are at their lowest level) and infections. The nurse in-structs the
patient and family on injection technique and about symptoms that require
follow-up with a physician (Chart 48-5).
Taking time to explain side effects and possible solutions may alleviate some of the anxiety of women who feel uncom-fortable asking questions. The more informed a patient is about the side effects of chemotherapy and how to manage them, the better she can anticipate and deal with them Chemotherapy may negatively affect the patient’s self-esteem, sexuality, and sense of well-being. Combined with the stress of a potentially life-threatening diagnosis, these changes can be over-whelming.
Because many women are
distressed by financial con-cerns and time spent away from the family, nursing
support and teaching can reduce emotional distress during treatment. Important
aspects of nursing care include communicating, facilitating support groups,
encouraging patients to ask questions, and promoting trust in health care
providers. Adequate time must be scheduled for clin-ical appointments to allow
discussion and questions. Most women with breast cancer today are treated in a
multidisciplinary environ-ment, and referrals to the dietitian, social worker,
psychiatrist, or spiritual advisor can assist in dealing with many of the
issues of cancer treatment. In addition, numerous community supports and
advocacy groups are available to these patients and their families.
HORMONAL THERAPY
Decisions about hormonal therapy for breast cancer are based on the outcome of an estrogen and progesterone receptor assay of tumor tissue taken during the initial biopsy. The tissue requires special handling by laboratory technicians with expertise in assess-ment techniques. Normal breast tissue contains receptor sites for estrogen. About two thirds of breast cancers are estrogen depen-dent, or ER-positive (ER+). An ER+ assay indicates that tumor growth depends on estrogen supply; therefore, measures that re-duce hormone production may limit the progression of the disease, and these receptors can be considered prognostic indicators. ER+ tumors may grow more slowly in general than those that do not depend on estrogen (ER−); thus, having an ER+ tumor indicates a better prognosis. A value less than 3 fmol/mg is considered neg-ative. Values of 3 to 10 are questionable, and values greater than 10 are considered positive.
The greater the value, the more bene-ficial the
anticipated effect from hormone suppression can be. Patients with tumors that
are positive for both estrogen and progesterone (PR+) generally have a more
favorable prognosis than patients with tumors that are ER− and
PR−. Most progesterone-receptive tumors also have a
positive estrogen receptor status. The loss of progesterone receptors can be a
sign of advancing disease. Premenopausal women and perimenopausal women are
more likely to have non–hormone-dependent lesions; postmenopausal women are
likely to have hormone-dependent lesions.
Hormonal
therapy may include surgery to remove endocrine glands (eg, the ovaries,
pituitary, or adrenal glands) with the goal of suppressing hormone secretion.
Oophorectomy (removal of the ovaries) is one treatment option for premenopausal
women with estrogen-dependent tumors. Tamoxifen is the primary hor-monal agent
used in breast cancer treatment today. Anastrazole (Arimidex), letrozole
(Femara), leuprolide (Lupron), megestrol (Megace), diethylstilbestrol (DES),
fluoxymesterone (Halotestin), and aminoglutethimide (Cytadren) are other
hormonal agents used to suppress hormone-dependent tumors. Most of these agents
may be associated with menopausal symptoms such as vasomotor changes.
Hypercalcemia may also occur and may necessitate dis-continuing the agent.
These hormonal agents are described in Table 48-5.
BONE MARROW TRANSPLANTATION
Bone
marrow transplantation (BMT) involves removing bone marrow from the patient and
then administering high-dose chemotherapy. The patient’s bone marrow, spared
from the ef-fects of chemotherapy, is then reinfused intravenously. This
pro-cedure is usually performed in specialized transplantation centers, and
specific patient preparation, education, and support must be given throughout
the treatment course. In 1999, scientific mis-conduct was discovered in the
only study that showed a benefit (Hagmann, 2000), casting doubt on the role
that BMT may play in breast cancer treatment. The use remains controversial
outside of clinical trials, since studies are not clear as to the true benefits
in comparison to standard high-dose chemotherapy (Antman, 2001).
INVESTIGATIONAL THERAPY: THE FUTURE
Research
is underway to develop chemotherapeutic agents that modify multidrug resistance
and agents that enhance or modify standard chemotherapy. Research in breast
cancer treatment in-cludes the following areas: peripheral stem cell
transplants, on-cogenes (tumor genes that control cell growth), growth factors
(substances released by cancer cells to make the environment more conducive to
growth), monoclonal antibodies (synthetic antibodies that fight cancer cells),
biologic response modifiers (substances that help increase the body’s immune
system response), and vaccine studies.
Another
treatment modality that has shown promise is trastuzumab (Herceptin). This
monoclonal antibody was engi-neered from mouse antibodies and closely resembles
a human antibody. Herceptin binds with the HER2 protein, and this pro-tein
regulates cell growth, thus inhibiting tumor cell growth. For women with
metastatic breast cancer, about 25% to 30% of tu-mors overproduce HER2, and
this monoclonal antibody can slow growth and possibly stimulate the immune
response. In fall 1998, the FDA approved this agent for the treatment of
metastatic breast cancer. Research is ongoing, but the addition of this agent
to traditional chemotherapy has shown improved survival rates in clinical
trials (Capriotti, 2001). Further research and clinical experience will
demonstrate the potential of this drug in the treat-ment of breast cancer,
particularly on the role of Herceptin for women undergoing adjuvant therapy.
Related Topics