Chapter: Organic Chemistry: Aromatic chemistry
Aromaticity
AROMATICITY
Key Notes
Definition
Aromatic
compounds such as benzene are more stable than suggested from their structure.
They undergo reactions
which retain the
aromatic ring system, and behave
differently from alkenes or polyenes.
Huckel rule
Aromatic
compounds are cyclic and planar with sp2 hybridized atoms. They also
obey the Hückel rule and have 4n2 π electrons where n1, 2, 3, ... Aromatic
systems can be monocyclic or polycyclic, neutral, or charged.
Definition
The term aromatic was originally applied to
benzene-like structures because of The distinctive aroma of these compounds,
but the term now means something different
in modern chemistry.
Aromatic compounds undergo
distinctive reactions which set them apart from other functional groups.
They are highlyunsaturated
compounds, but unlike
alkenes and alkynes,
they are relatively unreactive and will tend to
undergo reactions which involve a retention of their unsaturation. We have
already discussed the reasons for the stability of benzenein. Benzene is a
six-membered ring structurewith three formal doublebonds (Fig. 1a). However,
the six π electrons involved are not localized betweenany two carbon atoms.
Instead, they are delocalized around the ring which results in an increased
stability. This is why benzene is often written with a circle in the center of
the ring to signify the delocalization of the six π electrons (Fig. 1b).
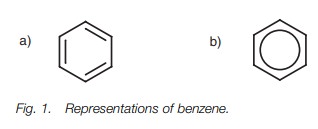
Reactions which disrupt this delocalization are not favored since it means a loss of stability, so benzene undergoes reactions where the aromatic ring system is retained. All six carbon atoms in benzene are sp2 hybridized, and the molecule itself is cyclic and planar – the planarity being necessary if the 2p atomic orbitals on each carbon atom are to overlap and result in delocalization.
Huckel rule
An aromatic molecule must be cyclic and planar
with sp2 hybridized atoms
(i.e. conjugated), but it must also obey what is known as the Hückel rule. This
rule states that the ring system must have 4n+2π electrons where n1, 2, 3, etc.
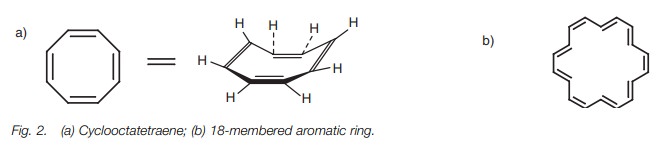
Therefore, ring systems which have 6, 10, 14,
... π electrons are aromatic. Benzene fits the Hückel rule since it has six
π electrons. Cyclooctatetraene has eight
π electrons and does not obey the Hückel rule. Although all the carbon atoms in
the ring are sp2
hybridized, cyclooctatetraene reacts like a conjugated alkene. It is not
planar, the π electrons are not delocalized and the molecule
consists of alternating single and double bonds (Fig. 2a). However, the 18-membered cyclic system (Fig.2b) does fit the Hückel rule (n=4) and is a planar molecule with
aromaticproperties and a delocalized π system.
It is also possible to get aromatic ions. The
cyclopentadienyl anion and the cycloheptatrienyl cation are both aromatic (Fig. 3). Both are cyclic and planar,
con-taining six π electrons, and all the atoms in the ring are sp2 hybridized.
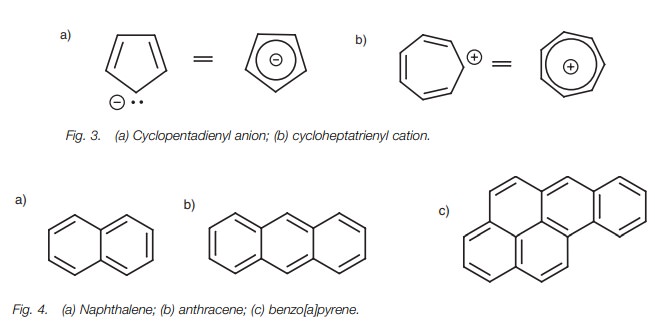
Bicyclic and polycyclic systems can also be aromatic (Fig. 4).
Related Topics