Chapter: Organic Chemistry: Aromatic chemistry
Electrophilic substitutions of benzene
ELECTROPHILIC SUBSTITUTIONS OF BENZENE
Key Notes
Definition
An
electrophilic substitution involves the substitution of one electrophile (a
proton) from the aromatic ring with another electrophile. The aromatic ring
remains intact.
Mechanism
The
mechanism of electrophilic substitution involves two stages. In stage 1, the
aromatic ring uses two of its π electrons to form a bond to the electrophile
which results in a positively charged intermediate. In stage 2, a proton is
lost from the ring and the electrons of the broken C–H bond are used to reform
the π bond
and restore aromaticity.
Intermediate stabilization
Electrophilic
substitution is aided by the fact that the positively charged intermediate is
stabilized by resonance, resulting in delocalization of the positive charge.
Since the intermediate is stabilized, the reaction takes place more readily.
Halogenation
Benzene
can be halogenated with chlorine and bromine. A Lewis acid such as FeBr3
or FeCl3 is required in order to activate the halogen and make it
more electrophilic.
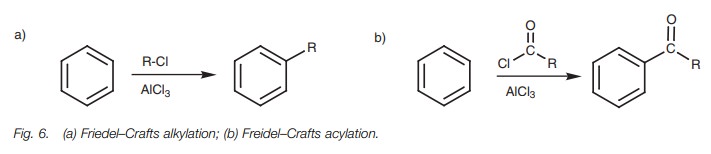
Friedel–Crafts alkylation and acylation
Alkyl
chains are linked to benzene by the Friedel–Crafts alkylation, using an alkyl
chloride and a Lewis acid. The Lewis acid is important in gener-ating a
carbocation which acts as the electrophile for the reaction. Primary alkyl
chlorides are not ideal for the Friedel–Crafts reaction since the pri-mary
carbocations generated can rearrange to more stable secondary or tertiary
carbocations. The Friedel–Crafts alkylation can also be carried out using an
alkene or an alcohol in the presence of a mineral acid. The Friedel–Crafts
acylation involves the reaction of benzene with an acid chloride and a Lewis
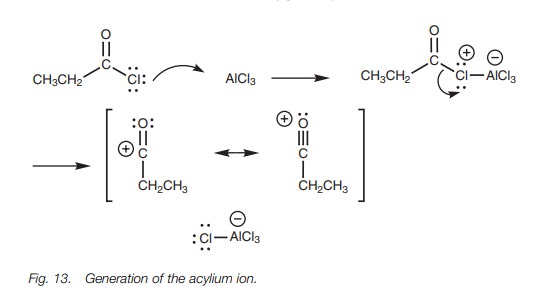
acid. An acylium ion is generated as the elec-trophile and has the advantage
over a carbocation in that it does not rearrange. The product is an aromatic
ketone. The ketone group can be reduced to give alkyl chains which would be
difficult to attach by the Friedel–Crafts alkylation.
Sulfonation and nitration
Benzene
is sulfonated with concentrated sulfuric acid. The reaction involves the
generation of sulfur trioxide which acts as the electrophile. Nitration is
carried out using concentrated nitric acid and sulfuric acid. The sulfuric acid
is present as an acid catalyst in the generation of the electrophilic
nitro-nium ion. Both electrophiles in these reactions are strong and a Lewis
acid is not required.
Definition
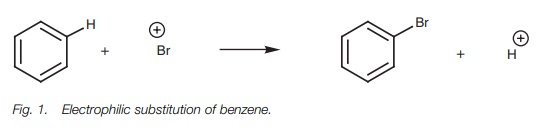
Aromatic rings undergo electrophilic
substitution, for example the bromination of
benzene (Fig. 1).
The reaction involves
an electrophile (Br+ ) replacing another electrophile
(H+ ) with the
aromatic ring remaining
intact. Therefore, one
electrophile replaces another and the reaction is known as an electrophilic
substitution. (At this stage we shall ignore how the bromine cation is formed.)
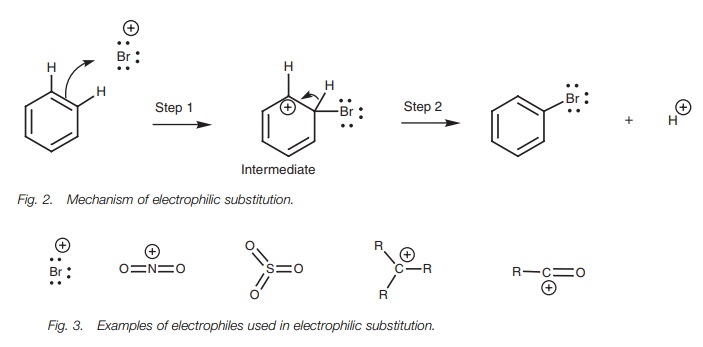
Mechanism
In the mechanism (Fig. 2) the aromatic ring acts as a nucleophile and provides two of
its π electrons to form a bond to Br . The aromatic
ring has now lost one of its formal double bonds resulting in a positively
charged carbon atom. This first step in the mechanism is the same as the one
described for the electrophilic addition to alkenes, and so the positively
charged intermediate here is equivalent to the carbocation intermediate in
electrophilic addition. However in step 2, the mechanisms of electrophilic
addition and electrophilic substitution differ. Whereas the carbocation
intermediate from an alkene reacts with a nucleophile to give an addition
product, the intermediate from the aromatic ring loses a proton. The C–H σ bond breaks and the two electrons move into the ring to reform the
π bond, thus regenerating the aromatic ring and neutralizing the
positive charge on the carbon. This is the mechanism undergone in all
electrophilic substitutions. The only difference is the nature of the
electrophile (Fig. 3).
Intermediate stabilization
The rate-determining step in electrophilic
substitution is the formation of the pos- itively charged intermediate, and so
the rate of the reaction is determined by the energy level of the transition
state leading to that intermediate. The transition state resembles the
intermediate in character and so any factor stabilizing the intermediate also
stabilizes the transition state and lowers the activation energy required for
the reaction. Therefore, electrophilic substitution is more likely to take
place if the positively charged intermediate can be stabilized. Stabilization
is possible if the positive charge can be spread amongst different atoms – a
process called delocalization. The
process by which
this can take
place is known
as resonance (Fig. 4).
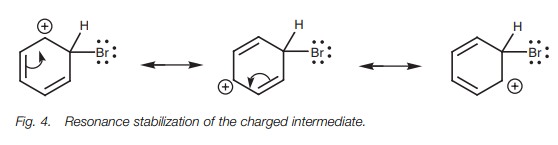
The resonance process involves two π electrons shifting their position round the ring to provide the
‘top’ carbon with a fourth bond and thus neutralize its posi-tive charge. In
the process, another carbon in the ring is left short of bonds and gains the
positive charge. This process can be repeated such that the positive charge is
spread to a third carbon. The structures drawn in Fig. 4 are known as resonance structures.
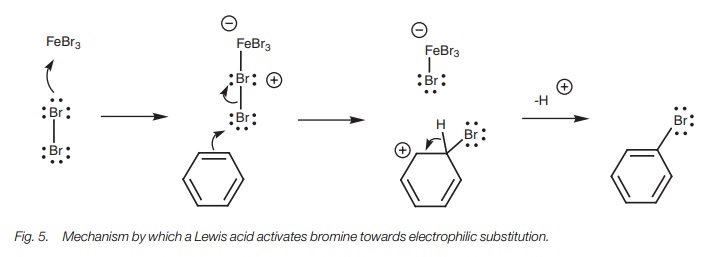
Halogenation
The stable aromatic ring means that aromatic
compounds are less reactive than alkenes to electrophiles. For example, an
alkene will react with Br2 whereas an aromatic ring will not.
Therefore, we have to activate the aromatic ring (i.e. make it a better
nucleophile) or activate the Br2 (i.e. make it a better
electrophile) if we want a reaction to occur. Laterly, we will explain how
electron-donating substituents on an aromatic ring increase the nucleophilicity
of the aromatic ring. Here, we shall see how a Br2 molecule can be
activated to make it a better electrophile. This can be done by adding a Lewis
acid such as FeCl3, FeBr3, or AlCl3 to the
reaction medium. These compounds all contain a central atom (iron or aluminum)
which is strongly electrophilic and does not have a full valence shell of
electrons. As a result, the central atom can accept a lone pair of electrons,
even from a weakly nucleophilic atom such as a halogen. In the example shown (Fig. 5) bromine uses a lone pair of
electrons to form a bond to the Fe atom in FeBr3 and becomes
positively charged. Bromine is now activated to behave as an electrophile and
will react more easily with a nucleophile (the aromatic ring) by the normal
mechanism for electrophilic substitution.
An aromatic ring can be chlorinated in a
similar fashion, using Cl2 in the presence of FeCl3.
Friedel–Crafts alkylation and acylation
Friedel–Crafts alkylation and acylation (Fig. 6) are
two other examples
of electrophilic substitution requiring the presence of a Lewis acid,
and are par- ticularly important because they allow the construction of larger
organic mol-ecules by adding alkyl (R) or acyl (RCO) side chains to an aromatic
ring.
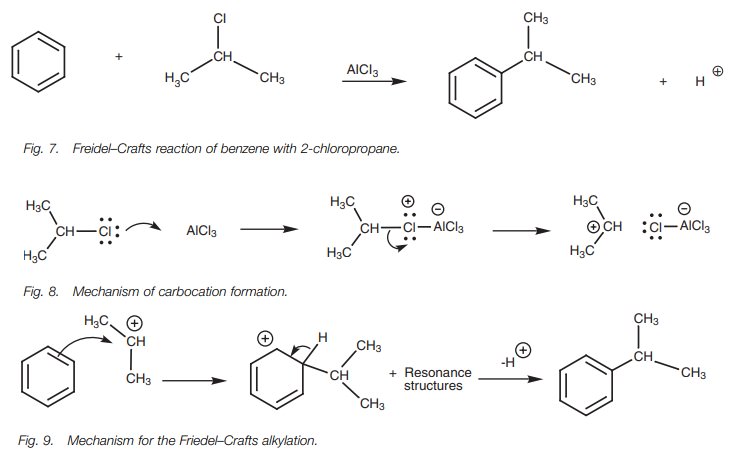
An example of Friedel–Crafts alkylation is the
reaction of benzene with 2-chloropropane (Fig.
7). The Lewis acid (AlCl3) promotes the formation of the
car-bocation required for the reaction and does so by accepting a lone pair of
electrons from chlorine to form an unstable intermediate which fragments to
give a carbo-cation and AlCl4- (Fig. 8). Once the carbocation is formed it reacts as an
elec-trophile with the aromatic ring by the electrophilic substitution
mechanism already described (Fig. 9).
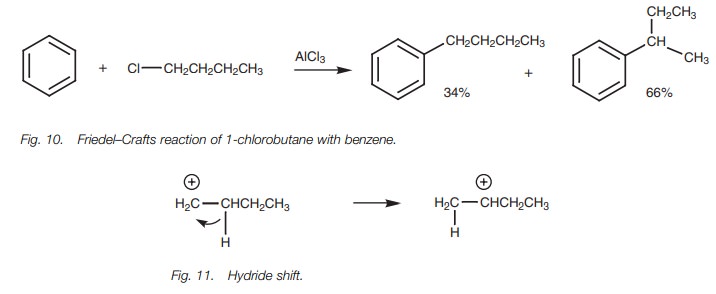
There are limitations to the Friedel–Crafts
alkylation. For example, the reaction of 1-chlorobutane with benzene gives two
products with only 34% of the desired product (Fig. 10). This is due to the fact that the primary carbocation
which is gen-erated can rearrange to a more stable secondary carbocation where
a hydrogen (and the two sigma electrons making up the C–H bond) ‘shift’ across
to the neigh-boring carbon atom (Fig. 11).
This is known as a hydride shift and
it takes place because the secondary carbocation is more stable than the
primary carbocation. Such rearrangements limit the type of alkylations which
can be carried out by the Friedel–Crafts reaction.
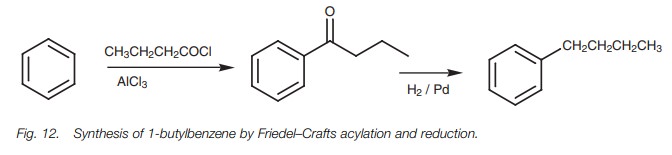
Bearing this in mind, how is it possible to
make structures like 1-butylbenzene in good yield? The answer to this problem
lies in the Friedel–Crafts acylation
(Fig.12). By reacting benzene with
butanoyl chloride instead of 1-chlorobutane, thenecessary 4-C skeleton is
linked to the aromatic ring and no rearrangement takes place. The carbonyl
group can then be removed by reducing it with hydrogen over a palladium
catalyst to give the desired product.
The mechanism of the Friedel–Crafts acylation
is the same as the Friedel–Crafts alkylation involving an acylium ion instead of a carbocation. As with the Friedel–Crafts
alkylation, a Lewis acid is required to generate the acylium ion (R–C=O)+
, but unlike a carbocation the acylium ion does not rearrange since there is
resonance stabilization from the oxygen (Fig.
13).
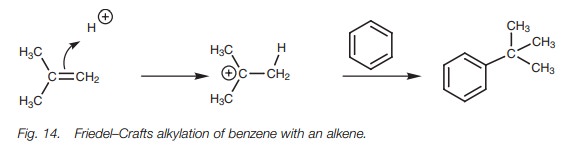
Friedel–Crafts alkylations can also be carried
out using alkenes instead of alkyl halides. A Lewis acid is not required, but a
mineral acid is. Treatment of the alkene with the acid leads to a carbocation
which can then react with an aromatic ring by the same electrophilic
substitution mechanism already described (Fig.
14). As far as the alkene is concerned, this is another example of
electrophilic addition where a proton is attached to one end of the double bond
and a phenyl group is added to the other.
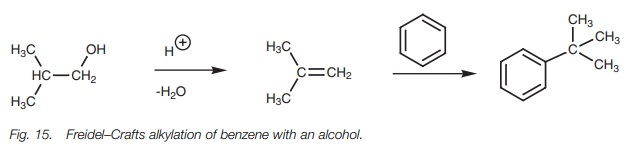
Friedel–Crafts reactions can also be carried
out with alcohols in the presence of mineral acid. The acid leads to the
elimination of water from the alcohol resulting in the formation of an alkene
which can then be converted to a carbocation as already described (Fig. 15).
Sulfonation and nitration
Sulfonation and nitration are electrophilic
substitutions which involve strong electrophiles and do not need the presence
of a Lewis acid (Fig. 16).
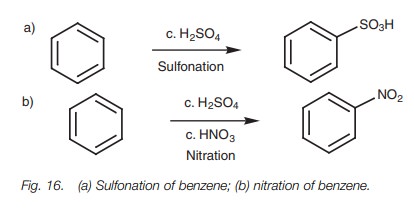
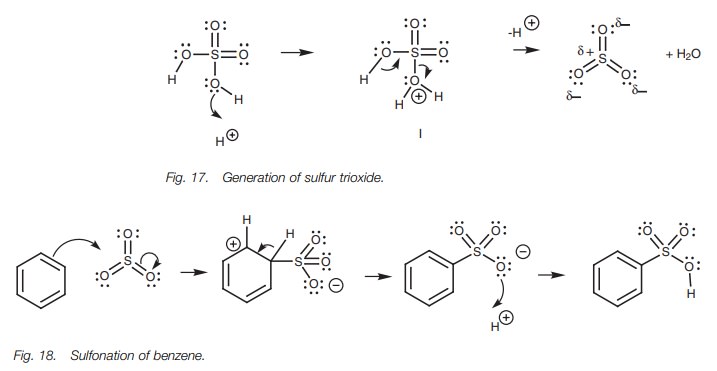
In sulfonation, the electrophile is sulfur
trioxide (SO3) which is generated under the acidic reaction
conditions (Fig. 17). Protonation of
an OH group generates a protonated intermediate (I). Since the oxygen gains a
positive charge it becomes a good leaving group and water is lost from the
intermedi-ate to give sulfur trioxide. Although sulfur trioxide has no positive
charge, it is a strong electrophile. This is because the sulfur atom is bonded
to three elec-tronegative oxygen atoms which are all ‘pulling’ electrons from
the sulfur, and making it electron deficient (i.e. electrophilic). During
electrophilic substitution (Fig. 18),
the aromatic ring forms a bond to sulfur and one of the π bonds between sulfur and oxygen is broken. Both electrons move to
the more electronegative oxy-gen to form a third lone pair and produce a
negative charge on that oxygen. This will finally be neutralized when the third
lone pair of electrons is used to form a bond to a proton.
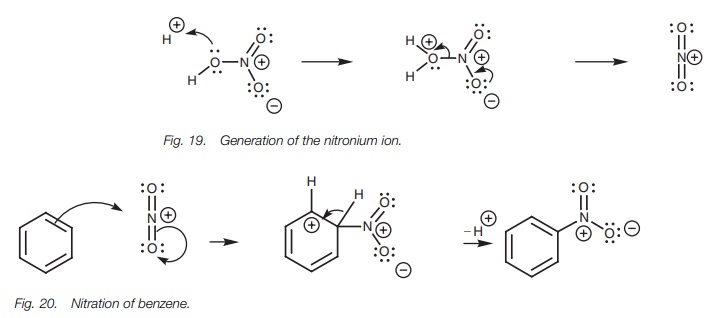
In nitration, sulfuric acid serves as an acid
catalyst for the formation of a nitronium ion (NO2 ) which is
generated from nitric acid by a very similar mechanism to that used in the
generation of sulfur trioxide from sulfuric acid (Fig. 19).
The mechanism for the nitration of benzene is very similar to sulfonation (Fig. 20). As the aromatic ring forms a bond to the electrophilic nitrogen atom, aπbond between N and O breaks and both electrons move onto the oxygen atom. Unlike sulfonation, this oxygen keeps its negative charge and does not pick up a proton. This is because it acts as a counterion to the neighboring positive charge on nitrogen.
Related Topics