Chapter: Organic Chemistry: Aromatic chemistry
Aromatic chemistry: Preparation and properties
PREPARATION AND PROPERTIES
Key Notes
Preparation
Simple
aromatic structures such as benzene, toluene, or naphthalene are isolated from
natural sources and converted to more complex aromatic structures.
Properties
Many
aromatic compounds have a characteristic aroma and burn with a smoky flame.
They are nonpolar, hydrophobic molecules which dissolve in organic solvents
rather than water. Aromatic molecules can interact by van der Waals
interactions or with a cation through an induced dipole interac-tion. Aromatic
compounds undergo reactions where the aromatic ring is retained. Electrophilic
substitution is the most common type of reaction. However, reduction is also
possible.
Spectroscopic analysis
Aromatic
compounds show characteristic absorptions in the IR spectrum due to ring
vibrations. Signals due to Ar-H stretching and bending may also be observed.
Signals for aromatic protons and carbons appear at character-istic positions
in nmr spectra. Fragmentation ions can
be observed in mass spectra which are characteristic of aromatic compounds.
Preparation
It is not practical to synthesize aromatic
structures in the laboratory from scratch and
most aromatic compounds
are prepared from
benzene or other
simple aromatic compounds (e.g. toluene and naphthalene). These in turn
are isolated from natural sources such as coal or petroleum.
Properties
Many aromatic compounds have a characteristic
aroma and will burn with a smoky flame. They are hydrophobic, nonpolar
molecules which will dissolve in organic solvents and are poorly soluble in
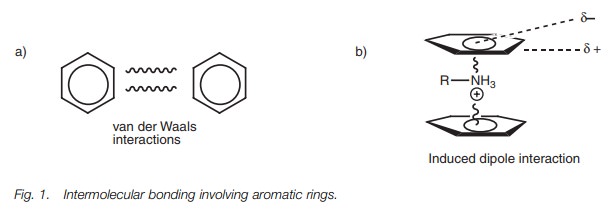
water. Aromatic molecules can interact with each other through intermolecular
bonding by van der Waals interactions (Fig.
1a). However, induced
dipole interactions are
also possible with
alkyl ammonium ions or metal ions where the positive charge of the
cation induces a dipole in the aromatic ring such that the face of the ring is
slightly negative and the edges are slightly positive (Fig. 1b). This results in the cation being sandwiched between two
aromatic rings.
Aromatic compounds are unusually stable and do not react in the same way as alkenes. They prefer to undergo reactions where the stable aromatic ring is retained. The most common type of reaction for aromatic rings is electrophilic substitution, but reduction is also possible.
Spectroscopic analysis
The
presence of an
aromatic ring can
be indicated by
IR, nmr and
mass spectroscopy.
In
the IR spectrum,
the stretching absorption
of an Ar-H
bond occurs at 3040–3010 cm−1 which
is higher than
the range for
an aliphatic C–H
stretch (3000–2800 cm−1). However, the absorption is usually
weak and may be hidden.
Absorptions due to ring vibrations are more
reliable and can account for up to four absorptions (typically about 1600,
1580, 1500 and 1450 cm−1). These occur at lower wavenumbers
to the C=C
stretching absorptions of
alkenes (1680– 1620 cm−1). Ar-H
out of plane
bending can give
absorptions in the
region 860–680 cm−1. The number and position of these
absorptions can indicate the sub- stitution pattern of the aromatic ring. For
example, an ortho disubstituted ring typ- ically has an absorption at 770–735
cm−1 whereas a para disubstituted ring
has an absorption in the region 860–800 cm−1. A monosubstituted aromatic ring
has two absorptions in the regions 690–710 and 730–770 cm−1 while a meta-disubstituted aromatic ring has
two absorptions in the regions 690–710 and 810–850 cm−1. These
bending absorptions are not always reliable and may be difficult to distinguish
from other absorptions in the region.
The
nmr signals for
aromatic protons and
carbons occur in
characteristic regions of the nmr spectra (typically 6.5–8.0 ppm for 1H
nmr; 110–160 ppm for 13C nmr). Moreover, it is possible to identify
the substitution pattern of the aro- matic ring based on the chemical shifts of
the signals. Tables exist which allow the calculation of the expected chemical
shifts based on the types of substituents that are present and their relative
positions on the ring. In the 1H spectrum, coupling patterns can often be
useful in determining substitution patterns for the aromatic ring. For example,
para-disubstituted aromatic rings often show two signals, both of which are
doublets.
There are characteristic fragmentation patterns
in the mass spectra of aromatic compounds.
For example, compounds
containing monosubstituted aromatic rings typically show fragmentation
ions at m/e 39, 50, 51, 52, & 77. Benzylic compounds usually have a strong
signal at m/e 91 due to cleavage of a benzylic fragmentation ion.
Since
aromatic rings contain
a conjugated π
electron system, they
can be detected by uv
spectroscopy. They generally show an intense absorption near 205 nm and a less
intense absorption in the range 255–275 nm.
Related Topics