Chapter: Organic Chemistry: Aromatic chemistry
Synthesis of mono-substituted benzenes
SYNTHESIS OF MONO-SUBSTITUTED BENZENES
Key Notes
Functional group transformations
Many
functional groups cannot be added directly to an aromatic ring by electrophilic
substitution but can be obtained by converting functional groups already added.
An amino group can be obtained by reduction of a nitro group then converted to
a large range of other functional groups. Alkyl groups can be oxidized to a
carboxylic acid group which can in turn be converted to other functional
groups.
Synthetic planning
When
planning the synthesis of an aromatic compound, it is best to work backwards
from the product in simple stages (retrosynthesis). If the sub-stituent present
cannot be added directly to the aromatic ring, it is best to consider what
functional group could be transformed to give the desired substituent.
Functional group transformations
Some substituents cannot be introduced directly
onto an aromatic ring by elec-trophilic substitution. These include the
following groups: –NH2, –NHR, NR2, NHCOCH3, CO2H,
CN, OH. Although these groups cannot be added directly onto the aromatic ring
they can be obtained by transforming a functional group which can be applied directly by
electrophilic substitution. Three of the most importanttransformations are
shown (Fig. 1).
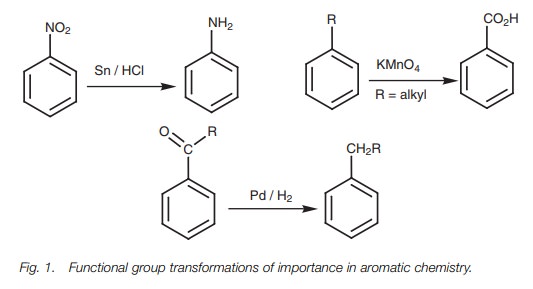
Nitro, alkyl, and acyl groups can readily be
added by electrophilic substitution and can then be converted to amino, carboxylic
acid, and alkyl groups respec-tively. Once the amino and carboxylic acid groups
have been obtained, they can be further converted to a large range of other
functional groups such as secondary and tertiary amines, amides, diazonium
salts, halides, nitriles, esters, phenols, alcohols, and ethers.
Synthetic planning
A knowledge of the electrophilic substitutions
and functional group transforma-tions which are possible is essential in
planning the synthesis of an aromatic com-pound. When designing such a
synthesis, it is best to work backwards from the product and to ask what it could
have been synthesized from – a process called retrosynthesis. We can illustrate this by designing a synthesis of
an aromatic ester(Fig. 2). An ester
functional group cannot be attached directly by electrophilic sub-stitution, so
the synthesis must involve several steps. The usual way to make an ester is
from an acid chloride which is synthesized in turn from a carboxylic acid.
Alternatively, the ester can be made directly from the carboxylic acid by
treating it with an alcohol and an acid catalyst. Either way, benzoic acid is
required to synthesize the ester. Carboxylic acids cannot be added directly to
aromatic rings either, so we have to look for a different functional group
which can be added directly, then transformed to a carboxylic acid. A carboxylic
acid group can be obtained from the oxidation of a methyl group. Methyl groups
can be added directly by Friedel–Crafts alkylation. Therefore a possible
synthetic route would be as shown in Fig.
2.

One possible problem with this route is the
possibility of poly-methylation in the first step. This is likely since the
product (toluene) will be more reactive than the starting material (benzene).
One way round this problem would be to use an excess of benzene.
As a second example, let us consider the
synthesis of an aromatic amine (Fig. 3).
The alkylamine group cannot be applied to an aromatic ring directly and so must
be obtained by modifying another functional group. Working backwards, the
alkylamine group could be obtained by alkylation of an amino group (NH2).
An amino group cannot be directly applied to an aromatic ring either. However,
an amino group could be obtained by reduction of a nitro group. A nitro group can be applied directly to an aromatic
ring. Thus, the overall synthesis would be nitra-tion followed by reduction,
followed by alkylation.
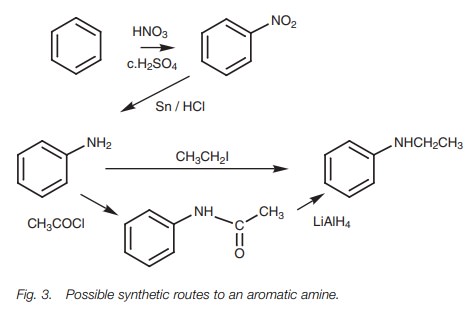
Note that there are two methods of converting aniline (PhNH2) to the final product. Alkylation is the direct method, but sometimes acylation followed by reduction gives better yields, despite the extra step. This is because it is sometimes difficult to control the alkylation to only one alkyl group.
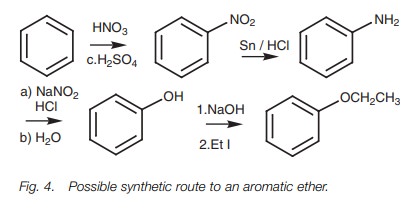
As our last example, we shall consider the
synthesis of an aromatic ether (Fig.4).
Here an ethoxy group is attached to the aromatic ring. The ethoxy group can-not
be applied directly to an aromatic ring, so we have to find a way of obtaining
it from another functional group. Alkylation of a phenol group would give the
desired ether, but a phenol group cannot be applied directly to the ring
either. However, we can obtain the phenol from an amino group, which in turn
can be obtained from a nitro group. The nitro group can be applied directly to
the ring and so the synthesis involves a nitration, reduction, conversion of
the amino group to a diazonium salt, hydrolysis, and finally an alkylation.
Related Topics