Chapter: Organic Chemistry: Aromatic chemistry
Synthesis of di- and tri-substituted benzenes
SYNTHESIS OF DI- AND TRI-SUBSTITUTED BENZENES
Key Notes
Di substituted benzenes
When
planning the synthesis of a di substituted benzene, it is important to consider
the directing properties of the two substituents. If an ortho/para-disubstituted
benzene is required, then the first group introduced should be ortho/para directing. If a meta-di
substituted benzene is required, the firstgroup introduced should be meta directing. In some cases, a
different group may have to be introduced in order to achieve the desired
substitution pat-tern and then transformed to the desired substituent.
Removable substituents
Two
common functional groups which can be removed from the ring are the amino and
the sulfonic acid groups. These groups can be used to direct or to block
substitution at particular locations in the ring. The sulfonic acid group is
particularly useful in obtaining ortho-di
substituted benzenes.
Di substituted benzenes
A full understanding of how substituents direct
further substitution is crucial in planning the synthesis of a di substituted
aromatic compound. For example, there are two choices which can be made in
attempting the synthesis of p-bromonitrobenzene
from benzene (Fig. 1). We could
brominate first then nitrate, or nitrate first then brominate. A knowledge of
how substituents affect elec-trophilic substitution allows us to choose the
most suitable route.
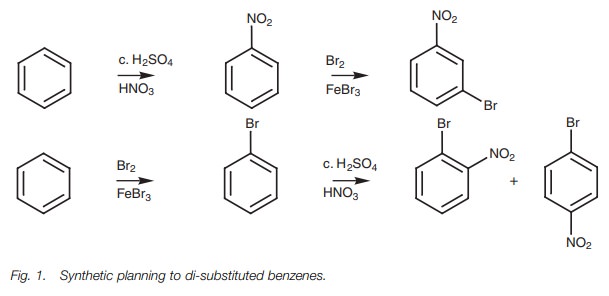
In the first method, nitrating first then
brominating would give predominantly the meta
isomer of the final product due to the meta
directing properties of the nitro group. The second method is better since the
directing properties of bromine are in our favor. Admittedly, we would have to
separate the para product from the ortho product, but we would still get a
higher yield by this route.
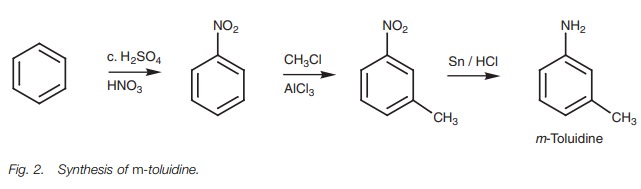
The synthesis of m-toluidine requires a little more thought (Fig. 2). Both the methyl and the amino substituents are activating
groups and direct ortho/para. However, the two substituents are meta with respect to each other. In
order to get metasubstitution we need
to introduce a substituent other than the methyl or nitrogroup which will
direct the second substitution to the meta
position. Moreover, once that has been achieved, the meta directing substituent has to be converted to one of the
desired substituents. The nitro group is ideal for this since it directs meta and can then be converted to the
required amino group.
This same strategy can be used for a large
range of meta-disubstituted aromatic
rings where both substituents are ortho/para directing since the nitro group can
be transformed to an amino group which can then be transformed to a large range
of different functional groups. Another tricky situation is where there are two
meta-directing substituents at ortho or para positions with respect to each other, for example, p-nitrobenzoic acid (Fig. 3). In this case, a methyl
substituent is added which is o/p directing. Nitration is then carried
out and the para isomer is separated
from any ortho isomer which might be
formed. The methylgroup can then be oxidized to the desired carboxylic acid.
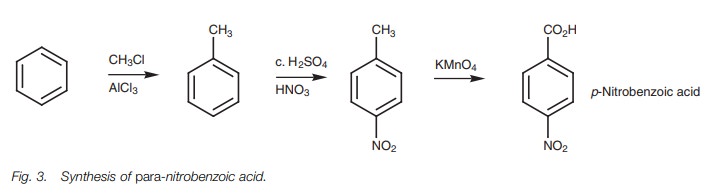
Larger alkyl groups could be used to increase the ratio of para to ortho substitu-tion since they can all be oxidized down to the carboxylic acid.
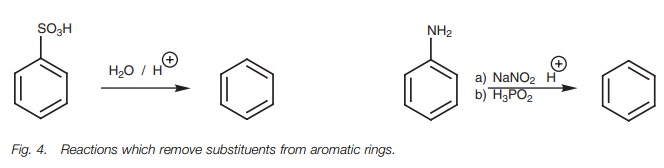
Removable substituents
It is sometimes useful to have a substituent present which can
direct or block a particular substitution, and which can then be removed once
the desired sub-stituents have been added. The reactions in Fig. 4 are used to remove substituents
from aromatic rings.
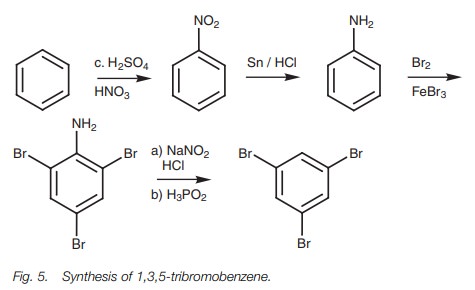
An example of how removable substituents can be
used is in the synthesis of 1,3,5-tribromobenzene (Fig. 5). This structure cannot be made directly from ben-zene by
bromination. The bromine atoms are in the meta
positions with respect to each other, but bromine atoms direct ortho/para. Moreover, bromine is a deactivating group and so it would be
difficult to introduce three such groups directly to benzene.
This problem can be overcome by using a strong
activating group which will direct ortho/para and which can then be removed at
the end of the synthesis. The amino group is ideal for this and the full
synthesis is shown in Fig. 5.
The synthesis of ortho-bromotoluene illustrates how a sulfonic acid can be used in a
synthesis. o-Bromotoluene could
conceivably be synthesized by bromination of toluene or by Friedel–Crafts
alkylation of bromobenzene (Fig. 6).
However, the reaction would also give the para-substitution
product and this is more likely if the electrophile is hindered from
approaching the ortho position by
unfavorable steric interactions. An alternative strategy would be to
deliberately substitute a group at the para
position of toluene before carrying out the bromination. This group would then
act as a blocking group at the para
position and would force the bromi-nation to take place ortho to the methyl group. If the blocking group could then be removed,
the desired product would be obtained. The sulfonic acid group is particularly
useful in this respect since it can be easily removed at the end of the
synthesis (Fig. 7).
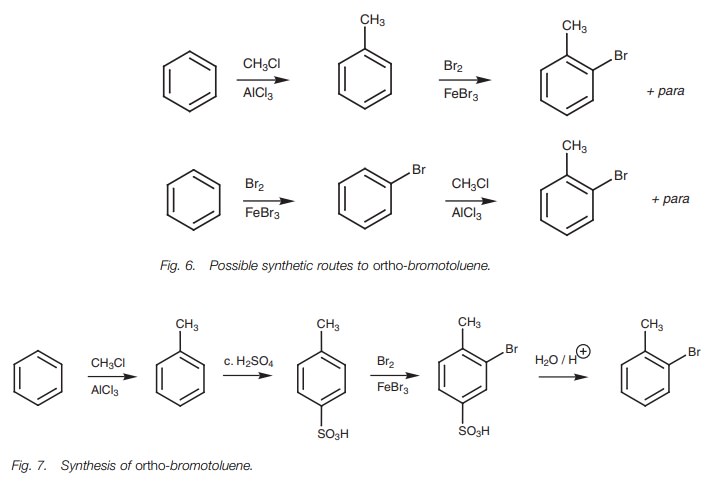
Note that the sulfonation of toluene could in theory take place at the ortho posi-tion as well as the para position. However, the SO3 electrophile is bulky and so the latter position is preferred for steric reasons. Once the sulfonic acid group is pre-sent, both it and the methyl group direct bromination to the same position (ortho to the methyl group meta to the sulfonic acid group).
Related Topics