Chapter: Organic Chemistry: Aromatic chemistry
Aromatic chemistry: Oxidation and reduction
OXIDATION AND REDUCTION
Key Notes
Oxidation
Aromatic
rings are resistant to oxidation but alkyl chains attached to the ring are not.
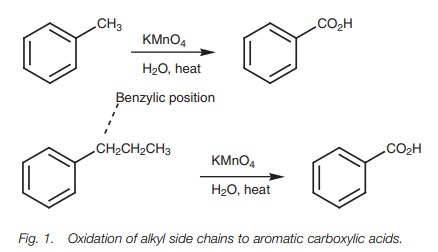
Alkyl substituents containing a benzylic hydrogen are oxidized to a carboxylic acid.
Reduction
The
aromatic ring is difficult to reduce with hydrogen and requires vigor- ous
reaction conditions using high pressure and heat, or strong catalysts such as
rhodium. Cyclohexane products are obtained. The resistance of the aromatic ring
to reduction allows the selective reduction of substituents such as ketones and
nitro groups without affecting the aromatic ring itself.
Oxidation
Aromatic rings are remarkably stable to
oxidation and are resistant to oxidizing agents such as potassium permanganate
or sodium dichromate. However, alkyl substituents on aromatic ring are
surprisingly susceptible to oxidation. This can be put to good use in the
synthesis of aromatic compounds since it is possible to oxidize an alkyl chain
to a carboxylic acid without oxidizing the aromatic ring. The mechanism of this
reaction is not fully understood, but it is known that a benzylic hydrogen has to be present (i.e. the carbon directly
attached to the ring must have a hydrogen). Alkyl groups lacking a benzylic
hydrogen are not oxidized.
Reduction
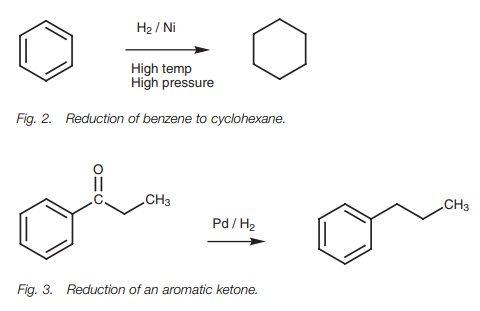
Aromatic rings can be hydrogenated to
cycloalkanes, but the reduction has to be carried out under strong conditions
using a nickel catalyst, high temperature and high pressure (Fig. 2) – much
stronger conditions than would be required for the is because of
the inherent stability
of
reduction of alkenes
. Thisaromatic rings. The reduction can also be
carried out using hydrogen and a platinum catalyst under high pressure, or with
hydrogen and a rhodium/carbon catalyst. The latter is a more powerful catalyst
and the reaction can be done at room temperature and at atmospheric pressure.
The resistance of the aromatic ring to
reduction is useful since it is possible to reduce functional groups which
might be attached to the ring without reducing the aromatic ring itself. For
example, the carbonyl group of an aromatic ketone can be reduced with hydrogen
over a palladium catalyst without affecting the aromatic ring (Fig. 3). This allows the synthesis of
primary alkylbenzenes which cannot be synthesized directly by the
Friedel–Crafts alkylation. It is worth noting that the aromatic ring makes the
ketone group more reactive to reduction than would normally be the case.
Aliphatic ketones would not be reduced under these conditions. Nitro groups can
also be reduced to amino groups under these conditions without affecting the
aromatic ring.
Related Topics