Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
Are naturally occurring pH buffers present in living organisms?
Are naturally occurring pH buffers present in living organisms?
Up until now, we have been considering buffers from the perspective of a chemist trying to control an experiment. However, the real importance of buffers is that they are critical to life. Buffer systems in living organisms and in the laboratory are based on many types of compounds. Because physiological pH in most organisms stays around 7, it might be expected that the phosphate buffer system would be widely used in living organisms. This is the case where phosphate ion concentrations are high enough for the buffer to be effective, as in most intracellular fluids.
The H2PO4/HPO4- pair is the principal buffer in cells. In blood, phosphate ion levels are inadequate for buffering, and a different system operates.
The buffering system in blood is based on the dissociation of carbonic acid (H2CO3):
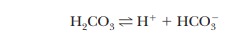
where the pKa of H2CO3 is 6.37. The pH of human blood, 7.4, is near the end of the buffering range of this system, but another factor enters into the situation.
Carbon dioxide can dissolve in water and in water-based fluids, such as blood. The dissolved carbon dioxide forms carbonic acid, which, in turn, reacts to produce bicarbonate ion:

At the pH of blood, which is about one unit higher than the pKa of carbonic acid, most of the dissolved CO2 is present as HCO3-. The CO2 being transported to the lungs to be expired takes the form of bicarbonate ion. A direct relationship exists between the pH of the blood and the pressure of carbon dioxide gas in the lungs. The properties of hemoglobin, the oxygen-carrying protein in the blood, also enter into the situation.
The phosphate buffer system is common in the laboratory (in vitro, outside the living body) as well as in living organisms (in vivo). The buffer system based on TRIS [tris(hydroxymethyl)aminomethane] is also widely used in vitro. Other buffers that have come into wide use more recently are zwitterions, which are compounds that have both a positive charge and a negative charge. Zwitterions are usually considered less likely to interfere with biochemical reactions than some of the earlier buffers (Table 2.8).
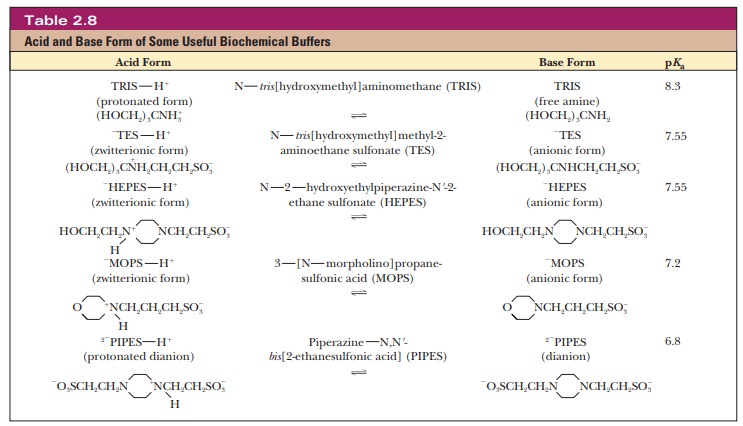
Most living systems operate at pH levels close to 7. The pKa values of many functional groups, such as the carboxyl and amino groups, are well above or well below this value. As a result, under physiological conditions, many important biomolecules exist as charged species to one extent or another. The practical consequences of this fact are explored in the following Biochemical Connections box.
Related Topics