Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
Solvent Properties of Water
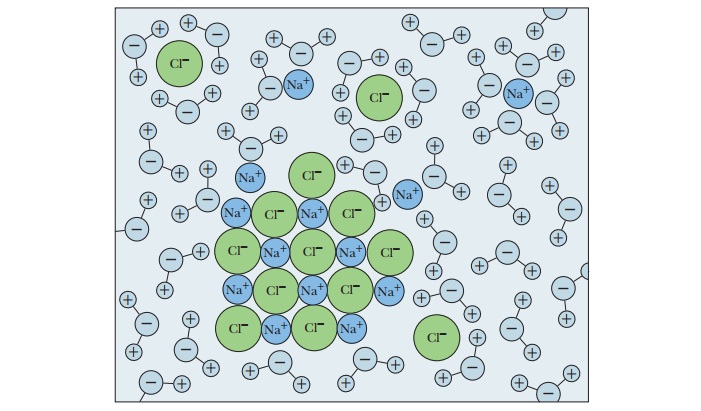
Solvent Properties of Water
Why do some chemicals dissolve in water while others do not?
The
polar nature of water largely determines its solvent properties. Ionic compounds with full charges, such
as potassium chloride (KCl, K+ and Cl- in
solution), and polar compounds with
partial charges (i.e., dipoles), such as ethyl alcohol (C2H5OH) or
acetone [(CH3)2C==O], tend to dissolve in water (Figures 2.2 and 2.3).
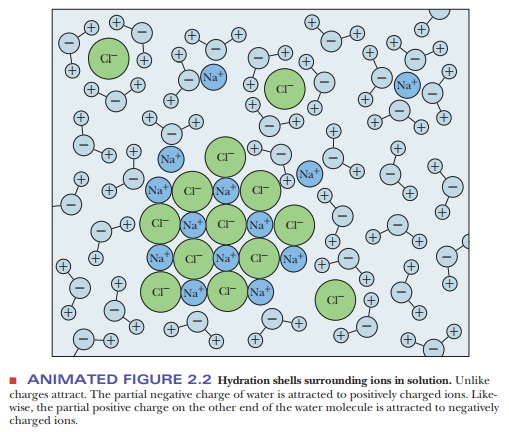
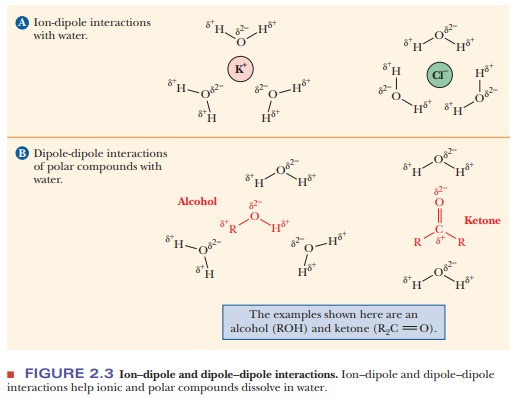
The underlying physical principle is electrostatic attraction between
unlike charges. The negative end of a water dipole attracts a positive ion or
the positive end of another dipole. The positive end of a water molecule
attracts a negative ion or the negative end of another dipole. The aggregate of
unlike charges, held in proximity to one another because of electrostatic
attraction, has a lower energy than would be possible if this interaction did
not take place. The lowering of energy makes the system more stable and more
likely to exist. These ion–dipole and
dipole–dipole interactions are
similar to the interactions between water molecules themselves in terms of the
quantities of energy involved. Examples of polar compounds that dissolve easily
in water are small organic molecules containing one or more electronegative
atoms (e.g., oxygen or nitrogen), including alcohols, amines, and carboxylic acids.
The attraction between the dipoles of these molecules and the water dipoles
makes them tend to dissolve. Ionic and polar substances are referred to as hydrophilic (“water-loving,” from the
Greek) because of this tendency.
Hydrocarbons
(compounds that contain only carbon and hydrogen) are nonpolar. The favorable
ion–dipole and dipole–dipole interactions respon-sible for the solubility of
ionic and polar compounds do not occur for nonpolar compounds, so these
compounds tend not to dissolve in water. The interac-tions between nonpolar
molecules and water molecules are weaker than dipolar interactions. The
permanent dipole of the water molecule can induce a temporary dipole in the
nonpolar molecule by distorting the spatial arrangements of the electrons in its
bonds. Electrostatic attraction is possible between the induced dipole of the
nonpolar molecule and the permanent dipole of the water mol-ecule (a dipole–induced dipole interaction), but
it is not as strong as that between permanent dipoles. Hence, its consequent
lowering of energy is less than that produced by the attraction of the water
molecules for one another. The associa-tion of nonpolar molecules with water is
far less likely to occur than the associa-tion of water molecules with
themselves.
A full
discussion of why nonpolar substances are insoluble in water requires the
thermodynamic arguments. However, the points made here about intermolecular
interactions will be useful background information for that discussion. For the
moment, it is enough to know that it is less favorable thermodynamically for
water molecules to be asso-ciated with nonpolar molecules than with other water
molecules. As a result, nonpolar molecules do not dissolve in water and are
referred to as hydrophobic
(“water-hating,” from the Greek). Hydrocarbons in particular tend to sequester
themselves from an aqueous environment. A nonpolar solid leaves undissolved
material in water. A nonpolar liquid forms a two-layer system with water; an
example is an oil slick. The interactions between nonpolar molecules are called
hydrophobic interactions or, in some
cases, hydrophobic bonds.
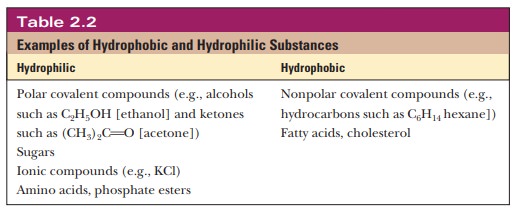
Table 2.2 gives examples of
hydrophobic and hydrophilic substances.
Why do oil and water mixed together separate into layers?
A single
molecule may have both polar (hydrophilic) and nonpolar (hydro-phobic)
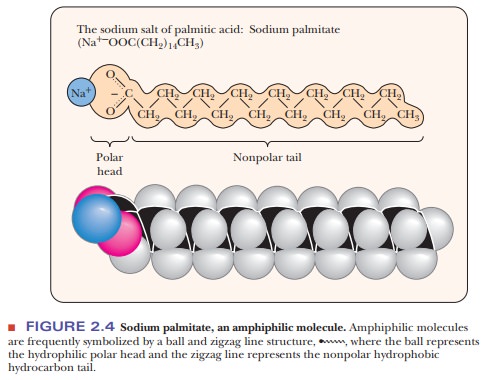
portions. Substances of this type are called amphipathic. A long-chain fatty acid having a polar carboxylic acid
group and a long nonpolar hydrocarbon portion is a prime example of an
amphipathic substance. The carboxylic acid group, the “head” group, contains
two oxygen atoms in addition to carbon and hydrogen; it is very polar and can
form a carboxylate anion at neutral pH. The rest of the molecule, the “tail,”
contains only carbon and hydrogen and is thus nonpolar (Figure 2.4). A compound
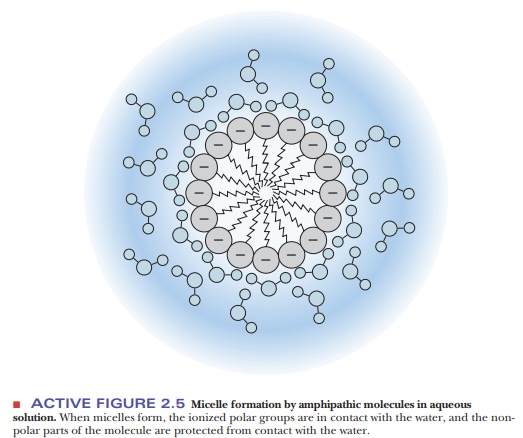
such as this in the presence of water tends to form structures called micelles, in which the polar head groups
are in contact with the aqueous environment and the nonpolar tails are
sequestered from the water (Figure 2.5). A similar process is responsible for
the separation of oil and water, such as you would see in Italian salad
dressing. When shaken, initially the substances mix. Immediately thereafter you
can see small spheres or oil droplets. As these float on water, they move to
the top and coalesce into the oil layer.
Interactions between nonpolar molecules themselves are very weak and depend on the attraction between short-lived temporary dipoles and the dipoles they induce. A large sample of nonpolar molecules will always include some molecules with these temporary dipoles, which are caused by a momen-tary clumping of bonding electrons at one end of the molecule. A temporary dipole can induce another dipole in a neighboring molecule in the same way that a permanent dipole does. The interaction energy is low because the association is so short-lived. It is called a van der Waals interaction (also referred to as a van der Waals bond). The arrangement of molecules in cells strongly depends on the molecules’ polarity, as we saw with micelles.
Summary
Water is a polar molecule, with a partial
negative charge on the oxygen and partial positive charges on the hydrogens.
Forces of attraction exist between the unlike
charges.
Polar substances tend to dissolve in water, but
nonpolar substances do not.
The properties of water have a direct effect on the behavior of biomolecules.
Related Topics