Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
How do we make buffers in the laboratory?
How do we make buffers in the laboratory?
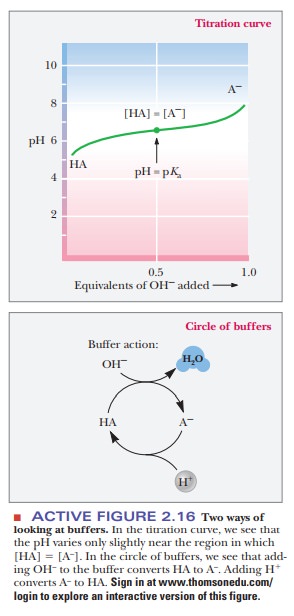
When we study buffers in theory, we often use the Henderson2Hasselbalch equation and do many calculations concerning ratios of conjugate base form to conjugate acid form. In practice, however, making a buffer is much easier. To have a buffer, all that is necessary are the two forms of the buffer present in the solution at reasonable quantities. This situation can be obtained by adding predetermined amounts of the conjugate base form (A-) to the acid form (HA), or we could start with one and create the other, which is how it is usually done in practice. Remember that HA and A- are interconverted by adding strong acid or strong base (Figure 2.16). To make a buffer, we could start with the HA form and add NaOH until the pH is correct, as determined by a pH meter. We could also start with A- and add HCl until the pH is correct. Depending on the relationship of the pH we desire to the pKa of the buffer, it may be more convenient to start with one than the other. For example, if we are making an acetic acid/acetate buffer at pH 5.7, it would make more sense to start with the A- form and to add a small amount of HCl to bring the pH down to 5.7, rather than to start with HA and to add much more NaOH to bring the pH up past the pKa.
Related Topics