Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
What is polarity?
What is polarity?
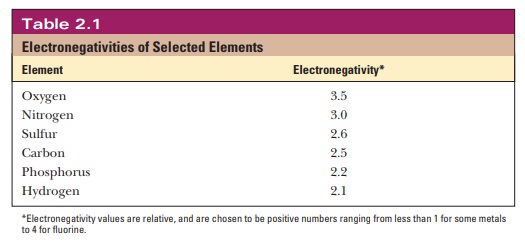
When two atoms with the same electronegativity
form a bond, the electrons are shared equally between the two atoms. However,
if atoms with differing electronegativity form a bond, the electrons are not
shared equally and more of the negative charge is found closer to one of the
atoms. In the O2H bonds in water, oxygen is more
electronegative than hydrogen, so there is a higher probability that the
bonding electrons are closer to the oxygen. The difference in electronegativity
between oxygen and hydrogen gives rise to a partial
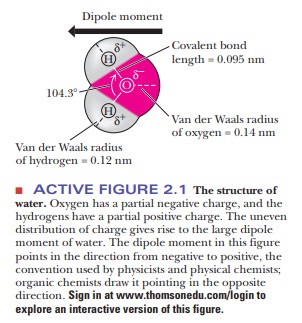
positive and negative charge, usually pictured asd1andd2, respectively (Figure 2.1). Bonds such as this
are called polar bonds. In
situations in which the electronegativity difference is quite small, such as in
the C-H bond in methane (CH4), the sharing of electrons in the bond
is very nearly equal, and the bond is essentially nonpolar.
The bonds in a molecule may be polar, but the
molecule itself can still be nonpolar because of its geometry. Carbon dioxide is
an example. The two C=O bonds are polar, but because the CO2
molecule is linear, the attraction of the oxygen for the electrons in one bond
is cancelled out by the equal and opposite attraction for the electrons by the
oxygen on the other side of the molecule.
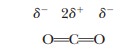
Water is a bent molecule with a bond angle of
104.3° (Figure 2.1), and the uneven sharing of electrons in the two bonds is
not cancelled out as it is in CO2. The result is that the bonding
electrons are more likely to be found at the oxygen end of the molecule than at
the hydrogen end. Bonds with positive and negative ends are called dipoles.
Related Topics