Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
Hydrogen Bonds
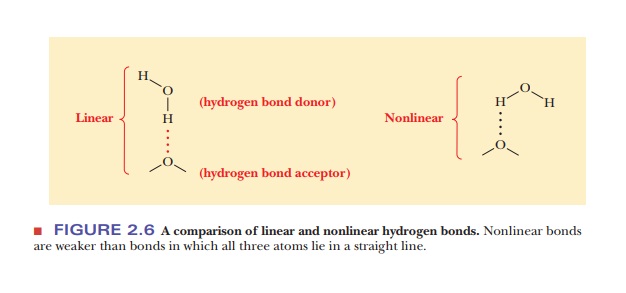
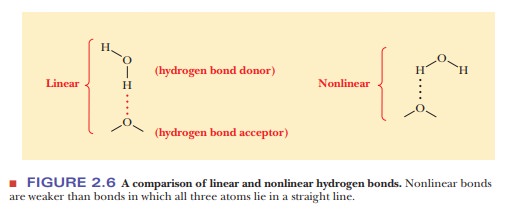
Hydrogen Bonds
In
addition to the interactions discussed, another important type of noncovalent
interaction exists: hydrogen bonding.
Hydrogen bonding is of electrostatic origin and can be considered a special
case of dipole–dipole interaction. When hydrogen is covalently bonded to a very
electronegative atom such as oxygen or nitrogen, it has a partial positive
charge due to the polar bond, a situation that does not occur when hydrogen is
covalently bonded to carbon. This partial positive charge on hydrogen can
interact with an unshared (nonbonding) pair of electrons (a source of negative
charge) on another electronegative atom. All three atoms lie in a straight
line, forming a hydrogen bond. This arrangement allows for the greatest
possible partial positive charge on the hydrogen and, consequently, for the
strongest possible interaction with the unshared pair of electrons on the
second electronegative atom (Figure 2.6). The group comprising the
electronegative atom that is covalently bonded to hydrogen is called the hydrogen-bond donor, and the
electronegative atom that contributes the unshared pair of electrons to the
interaction is the hydrogen-bondacceptor.
The hydrogen is not covalently bonded to the acceptor in the
usualdescription of hydrogen bonding.
Recent
research has cast some doubt on this view, with experimental evi-dence to
indicate some covalent character in the hydrogen bond.
Why does water have such interesting and unique properties?
A consideration of the hydrogen-bonding sites in HF, H2O, and NH3 can yield some useful insights. Figure 2.7 shows that water constitutes an optimum situ-ation in terms of the number of hydrogen bonds that each molecule can form. Water has two hydrogens to enter into hydrogen bonds and two unshared pairs of electrons on the oxygen to which other water molecules can be hydrogen-bonded.
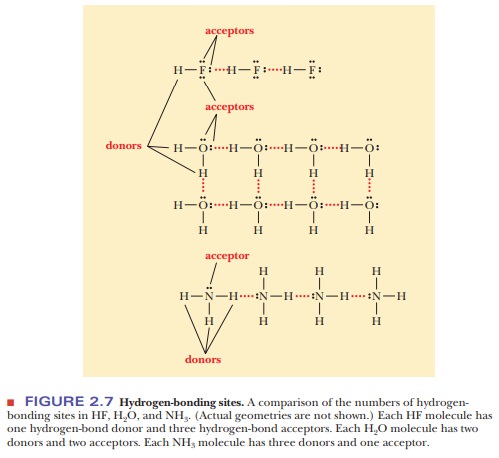
Each water molecule is involved in four hydrogen bonds-as a donor in two and as
an acceptor in two. Hydrogen fluoride has only one hydrogen to enter into a
hydrogen bond as a donor, but it has three unshared pairs of electrons on the
fluorine that could bond to other hydrogens. Ammonia has three hydrogens to
donate to a hydrogen bond but only one unshared pair of electrons, on the
nitrogen.
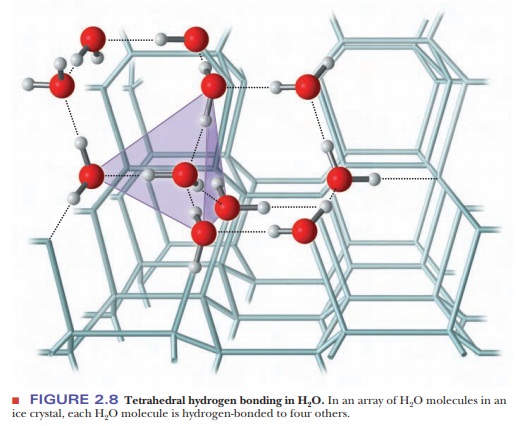
The
geometric arrangement of hydrogen-bonded water molecules has important
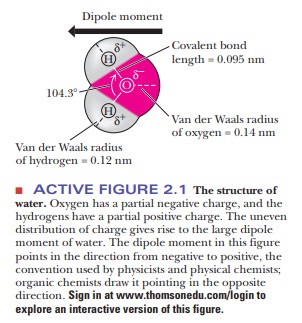
implications for the properties of water as a solvent. The bond angle in water
is 104.3°, as was shown in Figure 2.1, and the angle between the unshared pairs
of electrons is similar. The result is a tetrahedral arrange-ment of water
molecules. Liquid water consists of hydrogen-bonded arrays that resemble ice
crystals; each of these arrays can contain up to 100 water molecules. The
hydrogen bonding between water molecules can be seen more clearly in the
regular lattice structure of the ice crystal (Figure 2.8). There are several
differences, however, between hydrogen-bonded arrays of this type in liquid
water and the structure of ice crystals. In liquid water, hydrogen bonds are
constantly breaking and new ones are constantly forming, with some mol-ecules
breaking off and others joining the cluster. A cluster can break up and re-form
in 10210 to 10211 seconds
in water at 25°C. An ice crystal, in contrast, has a more-or-less-stable
arrangement of hydrogen bonds, and of course its number of molecules is many
orders of magnitude greater than 100.
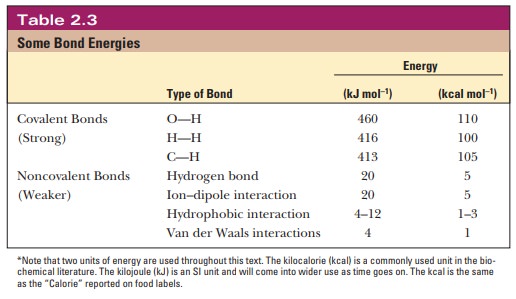
Hydrogen
bonds are much weaker than normal covalent bonds. Whereas the energy required
to break the O-H covalent bond is 460 kJ mol21 (110
kcal mol21), the energy of hydrogen
bonds in water is about 20 kJ mol21 (5 kcal
mol21) (Table 2.3). Even this
comparatively small amount of energy is enough to affect the properties of
water drastically, especially its melting point, its boil-ing point, and its
density relative to the density of ice. Both the melting point and the boiling
point of water are significantly higher than would be predicted
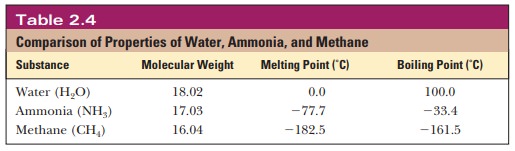
Other substances of about the same molecular
weight, such as methane and ammonia, have much lower melting and boiling
points. The forces of attraction between the molecules of these substances are
weaker than the attraction between water molecules, because of the number and
strength of their hydrogen bonds. The energy of this attraction must be
overcome to melt ice or boil water.
Ice has a lower density than liquid water because the fully hydrogen bonded array in an ice crystal is less densely packed than that in liquid water. Liquid water is less extensively hydrogen-bonded and thus is denser than ice. Thus, ice cubes and icebergs float. Most substances contract when they freeze, but the opposite is true of water.
In cold weather, the cooling systems of cars require
antifreeze to prevent freezing and expansion of the water, which could crack
the engine block. In laboratory procedures for cell fractionation, the same
principle is used in a method of disrupting cells with several cycles of
freezing and thawing. Finally, aquatic organisms can survive in cold climates
because of the density difference between ice and liquid water; lakes and rivers
freeze from top to bottom rather than vice versa.
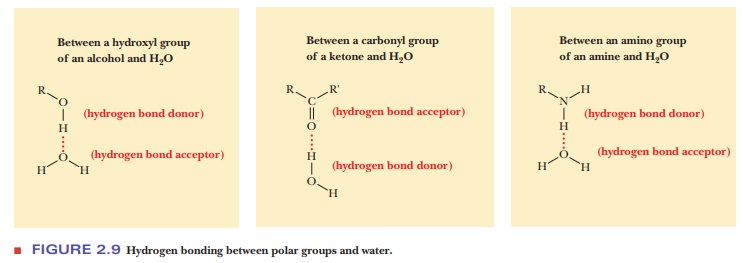
Hydrogen
bonding also plays a role in the behavior of water as a solvent. If a polar
solute can serve as a donor or an acceptor of hydrogen bonds, not only can it
form hydrogen bonds with water but it can also be involved in nonspe-cific
dipole2dipole interactions. Figure 2.9 shows some examples. Alcohols,
amines, carboxylic acids, and esters, as well as aldehydes and ketones, can all
form hydrogen bonds with water, so they are soluble in water. It is difficult
to overstate the importance of water to the existence of life on the Earth, and
it is difficult to imagine life based on another solvent. The following
Biochemical Connections box explores some of the implications of this
statement.
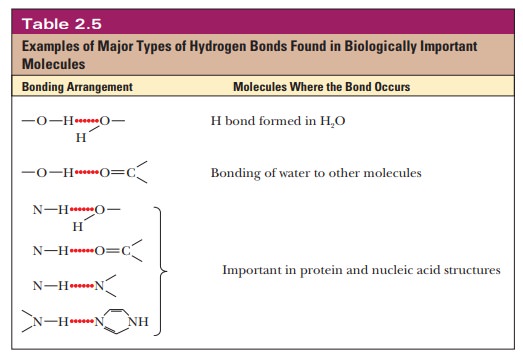
Other Biologically Important Hydrogen Bonds
Hydrogen
bonds have a vital involvement in stabilizing the three-dimensional structures
of biologically important molecules, including DNA, RNA, and proteins. The
hydrogen bonds between complementary bases are one of the most striking
characteristics of the double-helical structure of DNA. Transfer RNA also has a
complex three-dimensional structure characterized by hydrogen-bonded regions.
Hydrogen bonding in proteins gives rise to two important structures, the α-helix
and β-pleated sheet conformations. Both types of conformation are widely
encountered in proteins. Table 2.5 summarizes some of the most important kinds
of hydrogen bonds in biomolecules.
Summary
A hydrogen bond is a special example of a
dipole–dipole bond.
Water molecules are extensively hydrogen
bonded.
The ability to form strong
hydrogen bonds is responsible for the many unique characteristics of water,
such as its very high melting point and boiling point for a molecule of its
size.
The
three-dimensional structures of many important biomolecules, including proteins
and nucleic acids, are stabilized by hydrogen bonds.
Related Topics