Chapter: Biochemistry: Amino Acids and Peptides
Amino Acids Exist in a Three-Dimensional World
Amino Acids Exist in a
Three-Dimensional World
Why is it important to specify the three-dimensional structure of amino acids?
Among
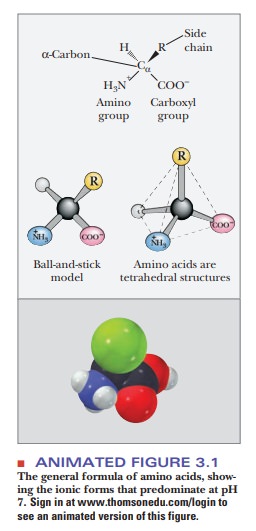
all the possible amino acids, only 20 are usually found in proteins. The
general structure of amino acids includes an amino group and a carboxylgroup,
both of which are bonded to thea-carbon (the one next to the
carboxylgroup). The α-carbon is also bonded to a hydrogen and to the side chain group, which is represented
by the letter R. The R group determines the identity of the particular amino
acid (Figure 3.1). The two-dimensional formula shown here can only partially
convey the common structure of amino acids because one of the most important
properties of these compounds is their three-dimensional shape, or stereochemistry.
Every
object has a mirror image. Many pairs of objects that are mirror imag-es can be
superimposed on each other; two identical solid-colored coffee mugs are an
example. In other cases, the mirror-image objects cannot be superim-posed on
one another but are related to each other as the right hand is to the left.
Such nonsuperimposable mirror images are said to be chiral (from the Greek cheir,
“hand”); many important biomolecules are chiral. A frequently encountered
chiral center in biomolecules is a carbon atom with four different groups
bonded to it (Figure 3.1). Such a center occurs in all amino acids except glycine.
Glycine has two hydrogen atoms bonded to the α-carbon; in other words, the side
chain (R group) of glycine is hydrogen. Glycine is not chiral (or,
alternatively, is achiral) because
of this symmetry. In all the other com-monly occurring amino acids, the α-carbon
has four different groups bonded to it, giving rise to two nonsuperimposable
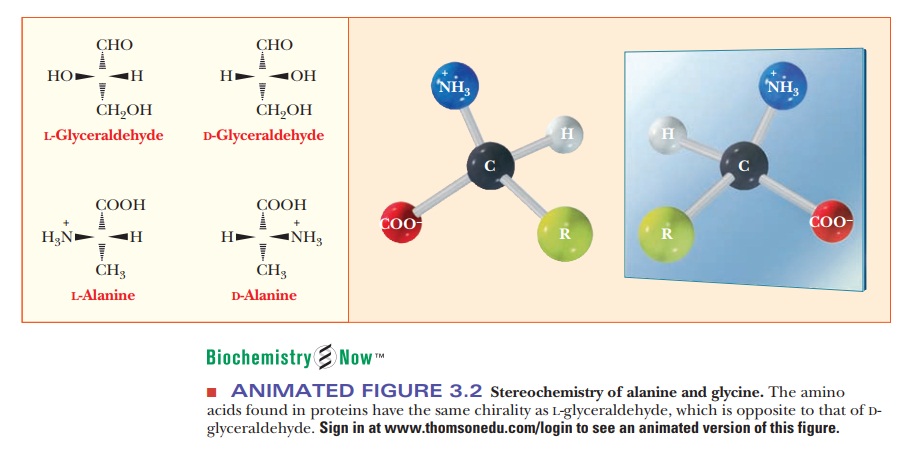
mirror-image forms. Figure 3.2 shows perspective drawings of these two
possibilities, or stereoisomers, for
ala-nine, where the R group is -CH3. The
dashed wedges represent bonds direct-ed away from the observer, and the solid
triangles represent bonds directed out of the plane of the paper in the
direction of the observer.
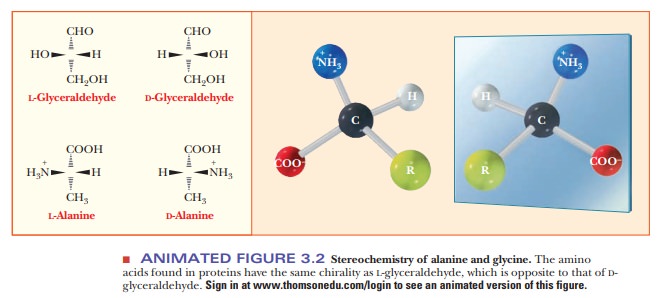
The two
possible stereoisomers of another chiral compound, L- and D-glycer-aldehyde, are shown for comparison with the corresponding
forms of alanine. These two forms of glyceraldehyde are the basis of the
classification of amino acids into L and D forms.
The terminology comes from the Latin laevus
and dexter, meaning “left” and
“right,” respectively, which comes from the ability ofoptically active compounds
to rotate polarized light to the left or the right. The two stereoisomers of
each amino acid are designated as L- andD-amino acids on the basis of their similarity to the
glyceraldehyde standard. When drawn in a certain orientation, the L form of glyceraldehyde has the hydroxyl group on the left side of
the molecule, and the D form has it on the right side, as shown in
per-spective in Figure 3.2 (a Fischer projection). To determine the L or D designation for an amino acid, it is drawn as
shown. The position of the amino group on the left or right side of the α-carbon
determines the L or D designation. The amino acids
that occur in proteins are all of the L form. Although D-amino acids occur in nature, most often in bacterial cell walls
and in some antibiotics, they are not found in proteins.
Summary
The amino acids that occur in proteins consist
of an amino group and a carboxyl group bonded to the same carbon atom. The
other two bonds of the carbon are to a hydrogen and to a side chain group,
shown as R in diagrams.

The
amino acids found in proteins are not superimposable on their mirror images
(with the exception of glycine). The mirror images known as L-amino acids are found in proteins; the D-amino
acid mirror image molecules are not.
Related Topics