Chapter: Biochemistry: Amino Acids and Peptides
Amino Acids Can Act as Both Acids and Bases
Amino Acids Can Act as Both Acids
and Bases
In a
free amino acid, the carboxyl group and amino group of the general structure
are charged at neutral pH-the carboxylate portion negatively and the amino
group positively. Amino acids without charged groups on their side chains exist
in neutral solution as zwitterions with no net charge. A zwitterion has equal
positive and negative charges; in solution, it is electrically neutral.
Neutral
amino acids do not exist in the form NH2-CHR-COOH
(that is, without charged groups).
What happens when we titrate an amino acid?
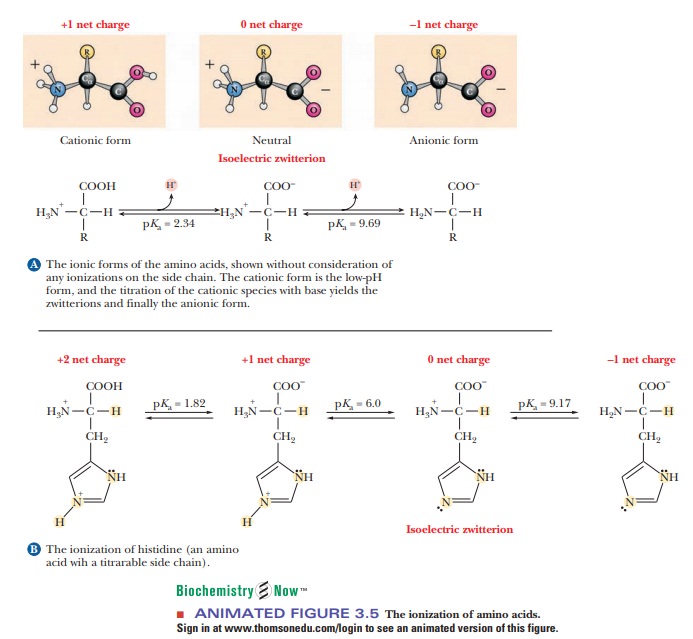
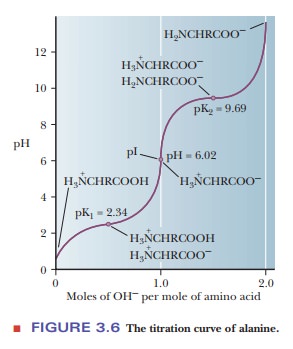
When an
amino acid is titrated, its titration curve indicates the reaction of each
functional group with hydrogen ion. In alanine, the carboxyl and amino groups
are the two titratable groups. At very low pH, alanine has a protonated (and
thus uncharged) carboxyl group and a positively charged amino group that is
also protonated. Under these conditions, the alanine has a net positive charge
of 1. As base is added, the carboxyl group loses its proton to become a
negatively charged carboxylate group (Figure 3.5a), and the pH of the solution
increases. Alanine now has no net charge. As the pH increases still further
with addition of more base, the protonated amino group (a weak acid) loses its
proton, and the alanine molecule now has a negative charge of 1. The titration
curve of alanine is that of a diprotic acid (Figure 3.6).
In
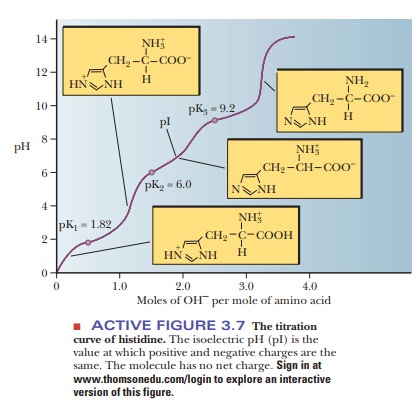
histidine, the imidazole side chain also contributes a titratable group. At
very low pH values, the histidine molecule has a net positive charge of 2
because both the imidazole and amino groups have positive charges. As base is
added and the pH increases, the carboxyl group loses a proton to become a
carboxylate as before, and the histidine now has a positive charge of 1 (Figure
3.5b). As still more base is added, the charged imidazole group loses its
proton, and this is the point at which the histidine has no net charge. At still
higher values of pH, the amino group loses its proton, as was the case with
alanine, and the histidine molecule now has a negative charge of 1. The
titration curve of histidine is that of a triprotic acid (Figure 3.7).
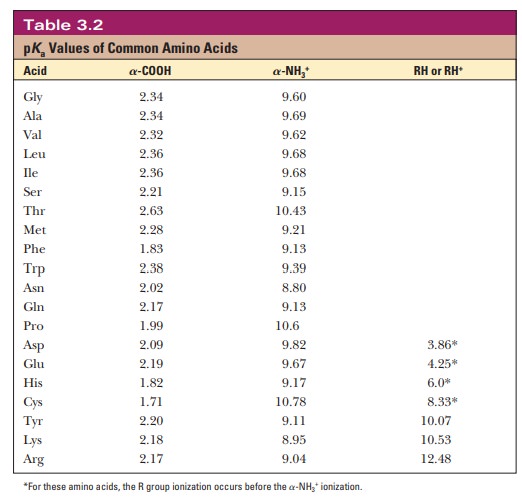
Like the acids we discussed, the titratable groups of each of the amino acids have characteristic pKa values. The pKa values of α-carboxyl groups are fairly low, around 2. The pKa values of amino groups are much higher, with values ranging from 9 to 10.5. The pKa values of side-chain groups, including side-chain carboxyl and amino groups, depend on the groups’ chemical nature. Table 3.2 lists the pKa values of the titratable groups of the amino acids. The clas-sification of an amino acid as acidic or basic depends on the pKa of the side chain as well as the chemical nature of the group. Histidine, lysine, and arginine are considered basic amino acids because each of their side chains has a nitrogen-containing group that can exist in either a protonated or deprotonated form. However, histidine has a pKa in the acidic range. Aspartic acid and glutamic acid are considered acidic because each has a carboxylic acid side chain with a low pKa value.
These groups can still be titrated after the amino acid is incorporated into a
peptide or protein, but the pKa of the
titratable group on the side chain is not necessarily the same in a protein as
it is in a free amino acid. In fact, it can be very different. For example, a pKa of 9
has been reported for an aspartate side chain in the protein thioredoxin.
The fact that amino acids, peptides, and proteins have different pKa values gives rise to the possibility that they can have different charges at a given pH. Alanine and histidine, for example, both have net charges of –1 at high pH, above 10; the only charged group is the carboxylate anion. At lower pH, around 5, alanine is a zwitterion with no net charge, but histidine has a net charge of 1 at this pH because the imidazole group is protonated. This property is useful in electrophoresis, a common method for separating molecules in an electric field. This method is extremely useful in determining the important proper-ties of proteins and nucleic acids. The pH at which a molecule has no net charge is called the isoelectric pH, or isoelectric point (given the symbol pI). At its isoelectric pH, a molecule will not migrate in an electric field. This property can be put to use in separation methods. The pI of an amino acid can be calculated by the following equation:
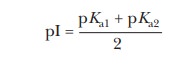
Most of the amino acids have only two pKa values, so this equation is easily used to calculate the pI. For the acidic and basic amino acids, however, we must be sure to average the correct pKa values. The pKa1 is for the functional group that has dissociated at its isoelectric point. If two groups are dissociated at isoelectric pH, the pKa1 is the higher pKa of the two. Therefore, pKa2 is for the group that has not dissociated at isoelectric pH. If there are two groups that are not dissociated, the one with the lower pKa is used. See the following apply your knowledge exercise.

Summary
The carboxyl group of every
amino acid is acidic, and the amino group is basic. The carboxylate group is
the conjugate base of the carboxyl group, and the protonated amino group is the
conjugate acid of the amino group. In addition, a number of side chains have
groups with acid–base properties.
Titration
curves can be obtained for amino acids, just as they can for any diprotic or
multiprotic acid. It is possible to determine the charge on amino acids at any
given pH.
Related Topics