Chapter: Biochemistry: Amino Acids and Peptides
The Peptide Bond
The Peptide Bond
Which groups on amino acids react to form a peptide bond?
Individual
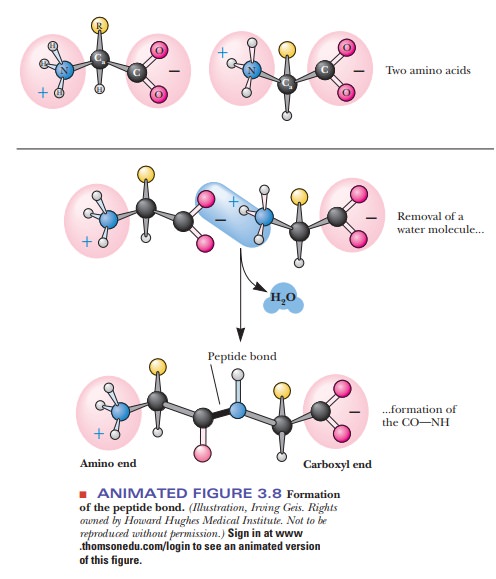
amino acids can be linked by forming covalent bonds. The bond is formed between
the α-carboxyl group of one amino acid and the α-amino group of the next one.
Water is eliminated in the process, and the linked amino acid residues remain after water is
eliminated (Figure 3.8). A bond formed in this way is called a peptide bond. Peptides are compounds
formed by linking small numbers of amino acids, ranging from two to several
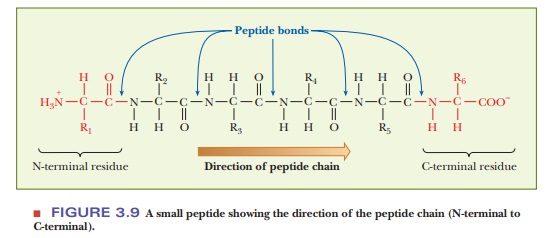
dozen. In a protein, many amino acids (usually more than a hundred) are linked
by peptide bonds to form a polypeptide
chain (Figure 3.9). Another name for a compound formed by the reaction
between an amino group and a carboxyl group is an amide.
The
carbon–nitrogen bond formed when two amino acids are linked in a peptide bond
is usually written as a single bond, with one pair of electrons shared between
the two atoms. With a simple shift in the position of a pair of electrons, it
is quite possible to write this bond as a double bond. This shift-ing of
electrons is well known in organic chemistry and results in resonance structures, structures that
differ from one another only in the positioning of electrons. The positions of
double and single bonds in one resonance structure are different from their
positions in another resonance structure of the same compound. No single
resonance structure actually represents the bonding in the compound; instead
all resonance structures contribute to the bonding situation.
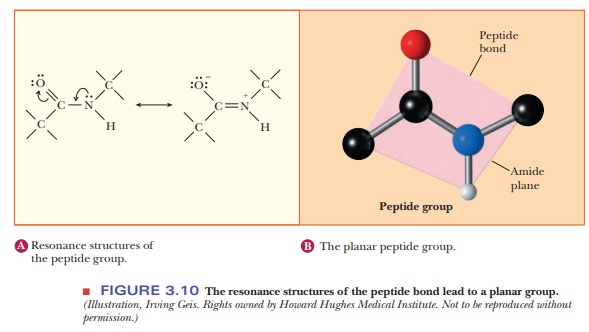
The
peptide bond can be written as a resonance hybrid of two structures (Figure
3.10), one with a single bond between the carbon and nitrogen and the other
with a double bond between the carbon and nitrogen. The peptide bond has
partial double bond character. As a result, the peptide group that forms the
link between the two amino acids is planar. The peptide bond is also stronger
than an ordinary single bond because of this resonance stabilization.
This
structural feature has important implications for the three-dimensional
conformations of peptides and proteins. There is free rotation around the bonds
between the α-carbon of a given amino acid residue and the amino nitro-gen and
carbonyl carbon of that residue, but there is no significant rotation around
the peptide bond. This stereochemical constraint plays an important role in
determining how the protein backbone can fold.
Summary
When the carboxyl group of
one amino acid reacts with the amino group of another to give an amide linkage
and eliminate water, a peptide bond is formed. In a protein, upward of a
hundred amino acids are so joined to form a polypeptide chain.
The
peptide group is planar as a result of resonance stabilization. This
stereochemical feature determines a number of features of the three-dimensional
structure of proteins.
Related Topics