Chapter: Biotechnology Applying the Genetic Revolution: Aging and Apoptosis
Apoptosis Involves a Proteolytic Cascade
APOPTOSIS
INVOLVES A PROTEOLYTIC CASCADE
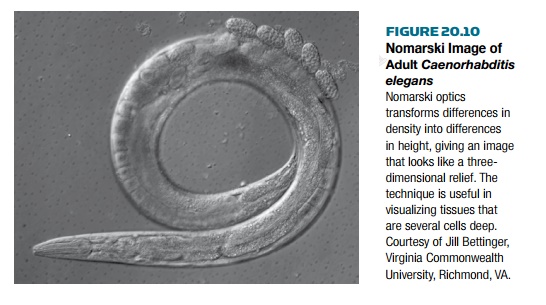
The first well-documented account of
apoptosis was seen in the nematode Caenorhabditis elegans . This small
worm is normally found in soil and eats soil bacteria. The worm is used as a
model organism for developmental genetics because it is easy to maintain, has
four developmental stages, and is transparent. Using a special microscope, the
Nomarski or differential interference microscope , every cell of C. elegans can
be seen ( Fig. 20.10 ).Using this technique, scientists have been able to trace
each cell division from the singlecelledegg through the entire adult. During
development, C. elegans generates 1090 cells, butthe final adult worm
has only 959. The other 131 cells die via apoptosis.
Many mutant worms were identified in
which the number of cells in the adult was more orless than 959, the wild-type
number. Four genes involved in aberrant apoptosis have beencharacterized. Three
of these are ced-3, ced-4 , and ced-9 (“ced” = cell death
abnormal), and thefourth is egl-1 (for egg laying defective). When the ced-3,
ced-4 , or egl-1 genes are defective, thereare more than 959 cells
in the adult worm. Thus these genes initiate or execute the apoptoticprogram.
When ced-9 is defective, there are fewer than 959 cells, indicating more
apoptosis thannormal. Thus, CED-9 protein inhibits apoptosis.
The CED and EGL proteins work in a
cascade that initiates cell death. That is, the action of one protein activates
the next protein, which in turn activates further proteins. Genetic and biochemical
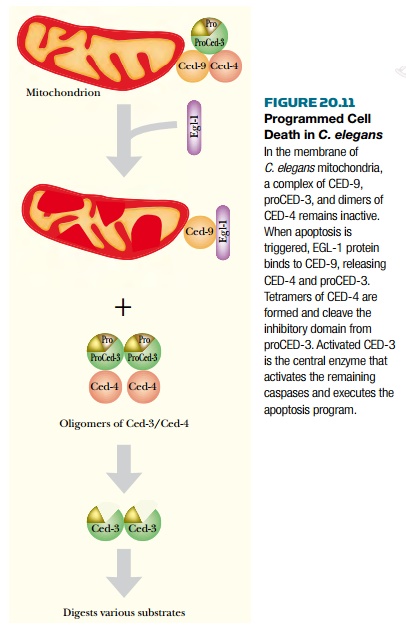
experiments have given the following model for apoptosis in C. elegans (
Fig. 20.11 ). The three CED proteins form an inactive complex in the membrane
of the mitochondrion. A signal from surrounding cells activates the synthesis
of EGL-1 protein. EGL-1 binds to CED-9 and removes it from the complex. This
activates CED-4, which is a protease that specifically cleaves a small
inhibitory domain from the end of CED-3. Activated CED-3 forms a heterotetramer
of two small and two large domains. This in turn digests various cellular
proteins by cutting after aspartic acid residues. This type of enzyme is known
as a caspase. Once CED-3 is active, it cleaves inhibitory domains off
other proteases, nucleases, and other caspases, thus executing the apoptotic
program.
Related Topics