Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies: From Structure to Therapeutic Application
Antibody Structure and Classes
ANTIBODY STRUCTURE AND CLASSES
Antibodies (Abs), immunoglobulin (Ig) are roughly Y-shaped molecules or
combinations of such molecules. There are five major classes of Ig’s: IgG,
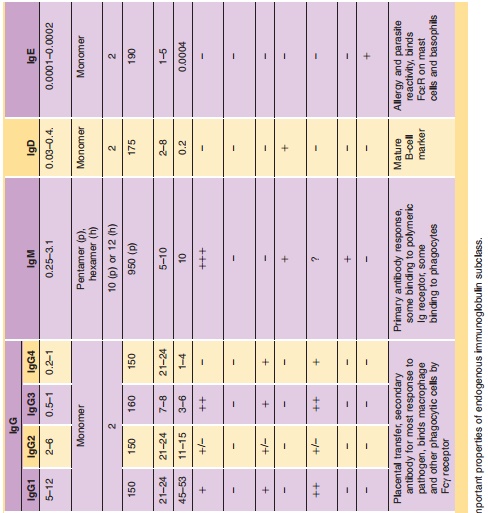
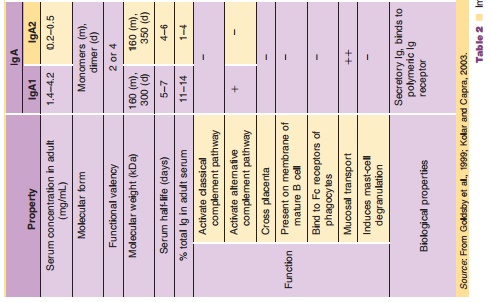
IgA, IgD, IgE, and IgM. Table 2 summarizes the character-istics of these
molecules, particularly their structure (monomer, dimer, pentamer, or hexamer),
molecular weight (ranging from ~150 kDa to ~1150 kDa), functions (e.g., activate
complement, FcγR binding). Among these classes, IgGs and their derivatives form the
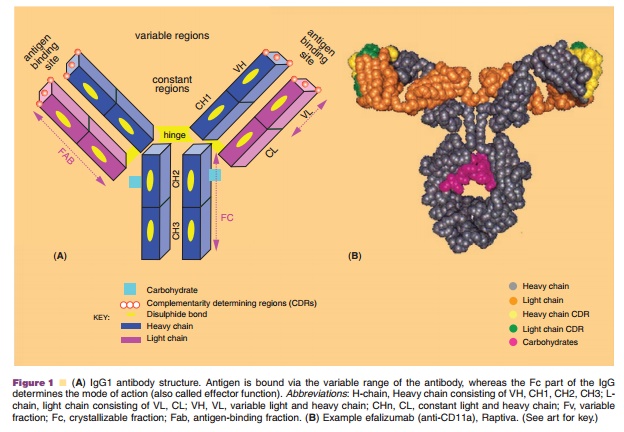
framework for the development of therapeutic antibodies. Figure 1 depicts the
general structure of an IgG with its structural components as well as a
conformational structure of efalizumab (anti-CD11a, Raptiva ). An IgG molecule
has four peptide chains, including two identical heavy (H) chains (~50–55 kDa)
and two identical light (L) chains (25 kDa), which are linked via disulfide
(S-S) bonds at the hinge region. The first ~110 amino acids of both chains form
the variable regions (VH and VL), and are also the antigen binding regions. Each V domain contains
three short stretches of peptide with hypervariable sequences (HV1, HV2, and
HV3), known as complementarity determining regions (CDRs), i.e., the region
that binds the antigen. The remaining sequences of each light chain consist of
a single constant domain (CL). The remainder of each heavy chain contains three constant regions (CH1, CH2, and CH3). Constant regions are
responsible for effector recognition and binding. IgG can be further divided
into four subclasses (IgG1, IgG2, IgG3, and IgG4). The differences among these
subclasses are also summarized in Table 2.
Related Topics