Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies: From Structure to Therapeutic Application
Antibody Derivatives [F(ab0)2, Fab, Antibody DrugConjugates (ADC)] and Fusion Proteins
Antibody Derivatives [F(ab0)2, Fab,
Antibody DrugConjugates (ADC)] and Fusion Proteins
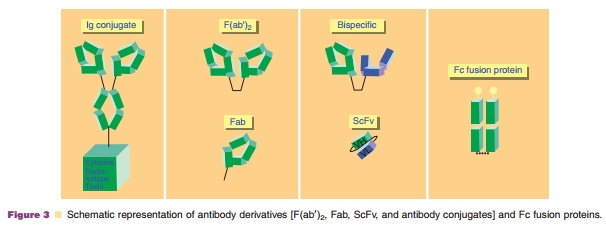
The fragments of antibodies (Fab, F(ab0)2, and scFv) have a shorter half-life compared with the full-sized
corresponding antibodies. scFv can be further engi-neered into a bivalent dimer
(diabody) (~60 kDa, or trimer: triabody ~90 kDa). Two diabodies can be further
linked together to generate bispecific tandem diabody (tandab). Figure 3
illustrates the structure of different antibody fragments. Of note, abciximab
and ranibizumab are two Fab fragments approved by FDA. Abciximab is a chimeric
Fab used for keeping blood from clotting and it exhibits a half-life of 20 to
30 minutes in serum and 4 hours in platelets (Schror and Weber, 2003).
Ranibizumab, which is admini-strated via an intravitreal injection, was
approved for the treatment of macular degeneration in 2006 and exhibits a
vitreous elimination half-life of 9 days (Albrecht and DeNardo, 2006).
The half-life of Fc fragments is more similar to that of full sized IgGs (Lobo et al., 2004). Therefore, Fc portions of IgGs have been used to form fusions with molecules such as cytokines, growth factor enzymes or the ligand-binding region of receptor or adhesion molecules to improve their half-life and stability. Alefacept, abatacept, and etanercept are three Fc-fusion proteins on the market. Etanercept, a dimeric fusion molecule consisting of the TNF-α receptor fused to the Fc region of human IgG1, has a half-life of approximately 70 to 100 hours (Zhou, 2005), which is much longer than the TNF-α receptor itself (30 minutes to 2 hours) (Watanabe et al., 1988).
Antibodies and antibody fragments can also be linked covalently with
toxic drugs or radioisotopes to form immunoconjugates or ADC. In each case, the
Ab is used as a delivery mechanism to selectively target the toxic drugs to
tumors. An example is gemtuzumab, an anti-CD33 mAb linked with a chemotherapy
drug ozogamicin. Ozogamicin itself has very significant side effects. It is
hypothesized that by virtue of targeting ozogamicin is mainly delivered to
cells expressing the CD33 protein with reduced exposure to normal cells. This
leads to an improved therapeutic window. The current radioimmunother-apy agents
licensed by the FDA are ibritumomab/ tiuxetan and tositumomab/131I-tositumomab for lym-phoma. Both
of the above intact mAbs bind CD-20 and carry a potent beta particle-emitting
radioisotope (9 0 Y for ibritumomab/tiuxetan and 1 31 I for tositumomab).
Related Topics