Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Thoracic Surgery
Anesthesia for Lung Resection: Anesthetic Considerations
ANESTHETIC CONSIDERATIONS
1. Preoperative Management
The majority of patients undergoing
pulmonary resections have underlying lung disease. It should be emphasized that
smoking is a risk factor for both chronic obstructive pulmonary disease and
coronary artery disease; both disorders commonly coexist in patients presenting
for thoracotomy.Echocardiography is useful for assessing baseline cardiac
function and may suggest evidence of cor pulmonale (right ventricular
enlargement or hyper-trophy) in patients with poor exercise tolerance. Stress
echocardiography (exercise or dobutamine) may be useful in diagnosing coronary
artery disease in patients with suggestive signs and symptoms.
Patients with tumors should be evaluated
for complications related to local extension of the tumor and paraneoplastic
syndromes (above). Preoperative chest radiographs and CT or MR images should be
reviewed. Tracheal or bronchial deviation can make tracheal intubation and
proper positioning of bronchial tubes much more difficult. Moreover, airway
compression can lead to difficulty in venti-lating the patient following
induction of anesthesia. Pulmonary consolidation, atelectasis, and large
pleu-ral effusions predispose to hypoxemia. The location of any bullous cysts
or abscesses should be noted.
Patients undergoing thoracic procedures
are at increased risk of postoperative pulmonary and car-diac complications. Perioperative
arrhythmias, par-ticularly supraventricular tachycardias, are thought to result
from surgical manipulations or distention of the right atrium following
reduction of the pul-monary vascular bed. The incidence of arrhythmias
increases with age and the amount of pulmonary resection.
2. Intraoperative Management
Preparation
As with anesthesia for cardiac surgery,
optimal prep-aration may help to prevent potentially catastrophic problems. The
frequent presence of poor pulmonary reserve, anatomic abnormalities, or
compromise of the airways, and the need for one-lung ventila-tion predispose
these patients to the rapid onset of hypoxemia. A well thought-out plan to deal
with potential difficulties is necessary. Moreover, in addi-tion to items for
basic airway management, special-ized and properly functioning equipment—such
as multiple sizes of single- and double-lumen tubes, a flexible (pediatric)
fiberoptic bronchoscope, a small-diameter “tube exchanger” of adequate length
to accommodate a double lumen tube, a continuous positive airway pressure
(CPAP) delivery system, and an anesthesia circuit adapter for administering
bronchodilators—should be immediately available.
Patients undergoing open-lung resections
(seg-mentectomy, lobectomy, pneumonectomy) often receive postoperative thoracic
epidural analgesia, unless there is a contraindication. However, patients are
increasingly being treated with numerous anti-platelet and anticoagulant
medications, which may preclude epidural catheter placement.
Venous Access
At least one large-bore (14- or
16-gauge) intrave-nous line is mandatory for all open thoracic surgi-cal
procedures. Central venous access (preferably on the side of the thoracotomy to
avoid the risk of pneumothorax on the side that will be ventilated intraoperatively),
a blood warmer, and a rapid infu-sion device are also desirable if extensive
blood loss is anticipated.
Monitoring
Direct monitoring of arterial pressure
is indicated for resections of large tumors (particularly those with
mediastinal or chest wall extension), and any procedure performed in patients
who have limited pulmonary reserve or significant cardiovascular disease.
Central venous access with monitoring of central venous pressure (CVP) is desirable for pneumonectomies and
resections of large tumors. Less invasive measures of cardiac output through
use of pulse contour analysis and transpulmo-nary thermodilution provide better
estimates of cardiac function and volume responsiveness. Pulmonary artery
catheters are very rarely used. Measurement of pulmonary artery pressures may
also not be accurate due to intrin-sic and extrinsic PEEP, lateral decubitus,
and open chest. In patients with significant coronary artery disease or
pulmonary hypertension, intraoperative monitoring can be enhanced by the use of
trans-esophageal echocardiography.
Induction of Anesthesia
After adequate preoxygenation, an
intravenous anesthetic is used for induction of most patients. The selection of
an induction agent should be based on the patient’s preoperative status. Direct
laryngoscopy should generally be performed only after adequate depth of
anesthesia has been achieved to prevent reflex bronchospasm and to obtund the
cardiovascular pressor response. This may be accomplished by incremental doses
of the induction agent, an opioid, or deepening the anesthesia with a volatile
inhalation agent (the latter is particularly useful in patients with reactive
airways).
Tracheal intubation with a single-lumen
tracheal tube (or with a laryngeal mask airway [LMA]) may be necessary, if the
surgeon performs diagnostic bron-choscopy (below) prior to surgery. Once the
bron-choscopy is completed, the single-lumen tracheal tube (or LMA) can be
replaced with a double-lumen bronchial tube (above). Controlled
positive-pressure ventilation helps prevent atelectasis, paradoxical breathing,
and mediastinal shift; it also allows control of the operative field to
facilitate the surgery.
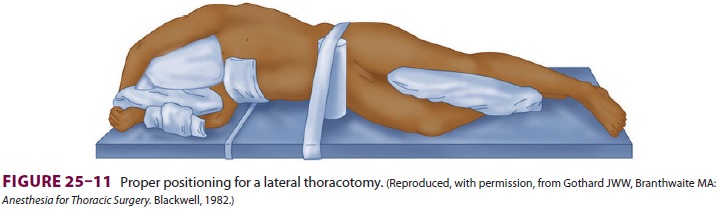
Positioning
Following induction, intubation, and
confirma-tion of correct tracheal or bronchial tube position, additional venous
access and monitoring may be obtained before the patient is positioned for
surgery. Most lung resections are performed with the patient in the lateral
decubitus position. Proper positioning avoids injuries and facilitates surgical
exposure. The lower arm is flexed and the upper arm is extended in front of the
head, pulling the scapula away from the operative field (Figure 25–11). Pillows
are placed between the arms and legs, and an axillary (chest) roll may be
positioned just beneath the dependent axilla to reduce pressure on the inferior
shoulder (it is assumed that this helps to protect the brachial plexus); care
is taken to avoid pressure on the eyes and the dependent ear.
Maintenance of Anesthesia
All current anesthetic techniques have been success-fully used for thoracic surgery, but the combination of a potent halogenated agent (isoflurane, sevoflu-rane, or desflurane) and an opioid is preferred by most clinicians. Advantages of the halogenated agents include: (1) potent dose-related bronchodila-tion; (2) depression of airway reflexes; (3) the ability to use a high inspired oxygen concentration (Fio 2), if necessary; (4) the ability to make relatively rapid adjustments in anesthetic depth; and (5) minimal effects on hypoxic pulmonary vasoconstriction . Halogenated agents generally have minimal effects on HPV in doses <1 minimum alveolar con-centration (MAC). Advantages of an opioid include:generally minimal hemodynamic effects;depression of airway reflexes; and (3) residual 3 postoperative analgesia. If epidural opioids are used postoperatively, intravenous opioids should be limited during surgery to prevent excessive postoperative respiratory depression. Maintenance of neuromuscular blockade with a nondepolarizing neuromuscular blocker (NMB) during surgery facilitates rib spreading as well as anesthetic management. Intravenous fluids should generally be restricted in patients undergoing pul-monary resections. Excessive fluid administration in thoracic surgical patients has been associated with acute lung injury in the postoperative period. No fluid replacement for estimated “third space” losses should be administered during lung resection. Excessive fluid administration in the lateral decubi-tus position may promote a “lower lung syndrome” (ie, gravity-dependent transudation of fluid into the dependent lung). The latter increases intrapulmo-nary shunting and promotes hypoxemia, particularly during one-lung ventilation. Moreover, the collapsed lung may be prone to acute lung injury due to surgi-cal retraction during the procedure and possible ischemia–reperfusion injury. During lung resec-tions, the bronchus (or remaining lung tissue) is usu-ally divided with an automated stapling device. The bronchial stump is then tested for an air leak under water by transiently sustaining 30 cm of positive pressure to the airway. Prior to completion of chest closure, all remaining lung segments should be fully expanded manually under direct vision. Controlled mechanical ventilation is then resumed and contin-ued until chest tubes are connected to suction.
Management of One-Lung Ventilation
Although still an intraoperative
problem, hypox-emia has become less frequent due to better lung isolation
methods, ventilation techniques, and the use of anesthetic agents with less
detrimental effects on hypoxic pulmonary vasoconstriction. Attention has
currently shifted toward avoidance of acute lung injury (ALI). Fortunately, ALI
occurs infrequently, with an incidence of 2.5 % of all lung resections
combined, and an incidence of 7.9% after pneumo-nectomy. However, when it
occurs, ALI is associ-ated with a risk of mortality or major morbidity of about
40%.
Based on current data, it seems that
protective lung ventilation strategies may minimize the risk of acute lung
injury after lung resection. This ventila-tory strategy includes the use of
lower tidal volumes (6–8 mL/kg), routine use of PEEP (5–10 cm H2O), lower Fio2
(50% to 80%), lower ventilatory pres-sures (plateau pressure 25 cm H2O; peak
airway pressure 35 cm H2O) through the use of pressure-controlled ventilation
and permissive hypercapnia. The use of lower tidal volumes may lead to lung
derecruitment, atelectasis, and hypoxemia. Lung derecruitment may be avoided by
application of external PEEP and frequent recruitment maneuvers. Although PEEP
may prevent alveolar collapse and development of atelectasis, it may cause a
decrease in Pao2 due to diversion of blood flow away from the dependent, ventilated
lung and an increase in total shunt. Thus, PEEP must be customized to the
underlying disease of each patient, and a new appli-cation of PEEP will almost
never be the appropriate way to treat hypoxemia that occurs immediately after
the onset of one-lung ventilation. Patients with obstructive pathology may
develop intrinsic PEEP. In these patients, the application of external PEEP may
lead to unpredictable levels of total PEEP. Although the management of one-lung
ventilation has long included the use of 100% oxygen, evidence of oxygen
toxicity has accumulated both experimen-tally and clinically. Although there is
no convincing evidence that outcomes are worsened with the use of 100% oxygen,
some clinicians recommend titrating Fio2 to maintain the oxygen saturation
above 90%, especially in patients who have undergone adjuvant therapy and are
at risk of developing ALI. Although there is no unequivocal evidence that one
mode of ventilation may be more beneficial than the other, pressure-controlled
ventilation may diminish the risk of barotrauma by limiting peak and plateau
air-way pressures, and the flow pattern results in a more homogenous
distribution of the tidal volume and improved dead space ventilation.
At the end of the procedure, the
operative lung is inflated gradually to a peak inspiratory pressure of less
than 30 cm H2O to prevent disruption of the staple
line. During reinflation of the operative lung, it may be helpful to clamp the
lumen serving the dependent lung to limit overdistension.
Periodic arterial blood gas analysis is
helpful to ensure adequate ventilation. End-tidal CO2 mea-surement may not be reliable due to increased
dead-space and an unpredictable gradient between the arterial and end-tidal CO 2 partial pressure.
Management of Hypoxia
Hypoxemia during one-lung anesthesia
requires one or more of the following interventions:
Adequate position of the bronchial tube
(or bronchial blocker) must be confirmed, as its position relative to the
carina can change as a result of surgical manipulations or traction; repeat
fiberoptic bronchoscopy through the tracheal lumen can quickly detect this
problem. Both lumens of the tube should also be suctioned to exclude excessive
secretions or obstruction as a factor.
Increase Fio2 to 1.0
Recruitment maneuvers on the dependent,
ventilated lung may eliminate atelectasis and improve shunt.
Optimize PEEP to the dependent,
nonoperative lung.
Ensure adequate cardiac output and
adequate oxygen carrying capacity.
CPAP or blow-by oxygen to the operative
lung will decrease shunting and improve oxygenation. However, inflation of the
operative lung during VATS will make identification and visualization of
the lung structures difficult for the surgeon; therefore, such maneuvers should
be applied carefully and cautiously.
Two-lung ventilation should be
instituted for severe hypoxemia. If possible, pulmonary artery clamp can also
be placed during pneumonectomy to eliminate shunt.
In patients with chronic obstructive
lung disease, one should always be suspicious of pneumothorax on the dependent,
ventilated side as a cause of severe hypoxemia. This complication requires
immediate detection and treatment by aborting the surgical procedure,
reexpanding the operative lung, and immediately inserting a chest tube in the
contralateral chest.
Alternatives to One-Lung Ventilation
Ventilation can be stopped for short
periods if 100%oxygen is insufflated at a rate greater than oxygen consumption (apneic oxygenation) into an unob-structed
tracheal tube. Adequate oxygenation canoften be maintained for prolonged
periods, but pro-gressive respiratory acidosis limits the use of this technique
to 10–20 min in most patients. Arterial Pco2 rises 6 mm Hg in the
first minute, followed by a rise of 3–4 mm Hg during each subsequent minute.
High-frequency positive-pressure
ventilation and high-frequency jet ventilation have been used during thoracic
procedures as alternatives to one-lung ventilation. A standard tracheal tube
may be used with either technique. Small tidal volumes (<2 mL/kg) allow decreased lung excursion, which may
facilitate the surgery but still allow ventilation ofboth lungs. Unfortunately,
mediastinal “bounce”— a to-and-fro movement—often interferes with the surgery.
3. Postoperative Management
General Care
Most patients are extubated shortly
after surgery to decrease the risk of pulmonary barotrauma (par-ticularly
“blowout” [rupture] of the bronchial suture line). Patients with marginal
pulmonary reserve should remain intubated until standard extubation criteria
are met; if a double-lumen tube was used for one-lung ventilation, it should be
replaced with a regular single-lumen tube at the end of surgery. A catheter
guide (“tube exchanger”) should be used if the original laryngoscopy was
difficult (above).
Patients are observed in the
postanesthesia care unit, and, in most instances, at least overnight or longer
in an intensive care unit or intermediate care unit. Postoperative hypoxemia
and respiratory aci-dosis are common. These effects are largely caused by
atelectasis and “shallow breathing (‘splinting’)” due to incisional pain.
Gravity-dependent transuda-tion of fluid into the intraoperative dependent lung
may also be contributory. Reexpansion edema of the collapsed nondependent lung
can also occur.
Postoperative hemorrhage complicates
about 3% of thoracotomies and may be associatedwith up to 20% mortality. Signs
of hemorrhage include increased chest tube drainage (>200 mL/h), hypotension, tachycardia, and a falling
hematocrit. Postoperative supraventricular tachyarrhythmias are common and
usually require immediate treat-ment. Routine postoperative care should include
maintenance of a semiupright (>30°) position, supplemental oxygen (40% to 50%),
incentive spi-rometry, electrocardiographic and hemodynamic monitoring, a
postoperative chest radiograph (to confirm proper position of all thoracostomy
tube drains and central lines and to confirm expansion of both lung fields),
and adequate pain relief.
Postoperative Analgesia
The importance of adequate pain
management in the thoracic surgical patient cannot be overstated. Inadequate
pain control in these high-risk patientswill result in splinting; poor
respiratory effort; and the inability to cough and clear secretions, with an
end result of airway closure, atelectasis, shunting, and hypoxemia.
Irrespective of the modality used, there must be a comprehensive plan for pain
management.
A balance between comfort and
respiratory depression in patients with marginal lung function is difficult to
achieve with parenteral opioids alone. Patients who have undergone thoracotomy
clearly benefit from the use of other techniques (described below) that may
reduce the need for parenteral opi-oids. If parenteral opioids are used alone,
they are best administered via a patient-controlled analgesia device.
In the absence of an epidural catheter,
intercos-tal or paravertebral nerve blocks with long-acting local anesthetics
may facilitate extubation, but have a limited duration of action, so
alternative means of pain management must be employed. Alternatively, a
cryoanalgesia probe may be used intraoperatively to freeze the intercostal
nerves (cryoneurolysis) and produce long-lasting anesthesia; unfortunately,
max-imum analgesia may not be achieved until 24–48 hr after the cryoanalgesia
procedure. Nerve regen-eration is reported to occur approximately 1 month after
the cryoneurolysis. Infusion of local anesthetic through a catheter placed in
the surgical wound dur-ing closure will markedly reduce the requirement for
parenteral opioids and improve the overall quality of analgesia relative to
parenteral opioids alone.
Epidural analgesia is the current
optimal method for acute pain control following thoracic surgical procedures.
It provides excellent pain relief, continuous therapy, and avoidance of the
side effects associated with administration of systemic opioids. On the other
hand, epidural techniques require attention from the acute pain team for the
duration of the infusion and subject the patient to the long list of
epidural-related side effects and complica-tions. However, there is still much
debate over the level of placement of the epidural catheter (tho-racic versus
lumbar), type of medication adminis-tered (opioid and/or local anesthetic), and timing of
medication administration (before surgical inci-sion vs before end of surgery).
Most practitioners use a combination of opioid (fentanyl, morphine,
hydromorphone) and local anesthetic (bupivacaine or ropivacaine), with the
epidural catheter placed at a thoracic level.
Postoperative Complications
Postoperative complications following
thoracotomy are relatively common, but fortunately most are minor and resolve
uneventfully. Blood clots and thick secretions may obstruct the airways and
result in atelectasis; suctioning may be necessary. Atelectasis is suggested by
tracheal deviation and shifting of the mediastinum to the operative side
fol-lowing segmental or lobar resections. Therapeutic bronchoscopy should be
considered for persistent atelectasis, particularly when associated with thick
secretions. Air leaks from the operative hemitho-rax are common following
segmental and lobar resections. Most air leaks stop after a few
days.Bronchopleural fistulae present as a sudden large air leak from the chest
tube that may beassociated with an increasing pneumothorax and partial lung
collapse. When they occur within the first 24–72 hr, they are usually the
result of inade-quate surgical closure of the bronchial stump. Delayed
presentation is usually due to necrosis of the suture line associated with
inadequate blood flow or infection.Some complications are rare, but deserve
spe-cial consideration because they can be life-threaten-ing and require
immediate exploratory thoracotomy. Postoperative bleeding was discussed above.
Torsion of a lobe or segment can occur as the remaining lung on the operative
side expands to occupy the hemithorax. The torsion usually occludes the
pul-monary vein to that part of the lung, causing venous outflow obstruction.
Hemoptysis and infarction can rapidly follow. The diagnosis is suggested by an
enlarging homogeneous density on the chest radio-graph and a closed lobar
orifice on bronchoscopy.
Acute herniation of the heart into the opera-tive
hemithorax can occur through the pericardial defect that may remain following a
pneumonectomy. A large pressure differential between the two hemithoraces is
thought to trigger this catastrophic event. Cardiac herniation into the right
hemithorax results in sudden severe hypoten-sion with an elevated CVP because of torsion of the central
veins. Cardiac herniation into the lefthemithorax following left pneumonectomy
results in sudden compression of the myocardium, result-ing in hypotension,
ischemia, and infarction. A chest radiograph shows a shift of the cardiac
shadow into the operative hemithorax.
Extensive mediastinal dissections can
injure the phrenic, vagus, and left recurrent laryngeal nerves. Postoperative
phrenic nerve palsy presents as eleva-tion of the ipsilateral hemidiaphragm
together with difficulty in weaning the patient from the ventilator. Large
chest wall resections may include part of the diaphragm, causing a similar
problem, in addition to a flail chest. Paraplegia rarely follows thoracot-omy
for lung resection. There are reports of cellulose gauze and other debris
migrating from the thoracic gutter into the spinal canal, resulting in spinal
cord compression. If an epidural catheter has been placed, any loss of motor
function or unexplained back pain should immediately trigger imaging to rule
out epi-dural hematoma.
Related Topics