Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : Anesthesia and the lung
Alveolar air equation
Alveolar air equation
The PO2 we expect to find in arterial blood depends, in large part, on the inspired concentration. Oxygen makes up approximately 21% of the volume of dry air. If we assume the ambient (sea level) pressure to be 760 mmHg, the partial pressure of oxygen in dry air would be 160 mmHg (760 × 0.21). The warm and moist airways add water (water vapor pressure is temperature-dependent, and at 37 °C it is 47 mmHg). Thus, breathing room air, our inspired oxygen concentration (PiO2) on its way through the nose and upper airway will be diluted by water vapor:
PiO2= (760 − 47) × 0.21 = 150 mmHg
Once it arrives in the alveolus, this inspired oxygen will be diluted by carbon dioxide and taken up into the bloodstream. Summarized mathematically,4 the resulting equation is too cumbersome for clinical application. Instead, we use the approximation commonly referred to as the alveolar air equation:
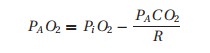
where the “A” subscript denotes alveolar gas.
If we take the trouble of calculating for a person breathing room air (PiO2= 150 mmHg), assuming R= 0.8 and PACO2= 40 mmHg, we arrive at a PAO2 of about 100 mmHg. You can easily see that many factors can change the results: altitude, retention or rebreathing of CO2, changing the concentration of oxygen in the inspired gas, changing the respiratory quotient, or changing the patient’s temperature.
Related Topics