Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : Anesthesia and the lung
Studies of pulmonary function
Studies of pulmonary function
Spirometry
Pulmonary
function tests (PFTs) are rarely indicated in preparation for anes-thesia,
though they can tell us whether a patient with severe lung disease has been
optimally prepared. Pulmonary restrictive and obstructive diseases worry us.
Short of treating infection, we cannot do much about restrictive disease;
how-ever, it can co-exist with obstructive bronchospasm, which is common and
can be treated with bronchodilators. Of the many pulmonary function studies, we
pay particular attention to forced vital capacity (FVC). FVC values below 15
mL/kg give rise to great concern. How much the patient can exhale in 1 second
(FEV1), and whether this can be improved by bronchodilators
determines obstructive
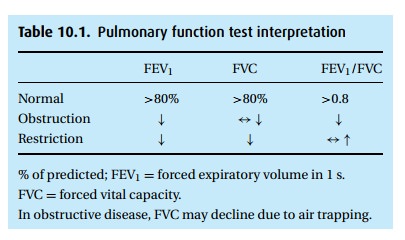
Typically, PFT results are reported as “% of predicted,” based on popula-tion
studies that consider the patient’s height, age, and gender. We accept values
of at least 80% predicted as normal. With obstructive disease, the patient
exper-iences airway closure during exhalation, measured as a low FEV1
(Table 10.1). With advanced disease and air
trapping, the FVC might also decline, though not as much as the FEV1,
thus the hallmark of obstructive disease is a reduced ratio of FEV1
to FVC. With age, this ratio declines as well (to perhaps 0.7), so again we
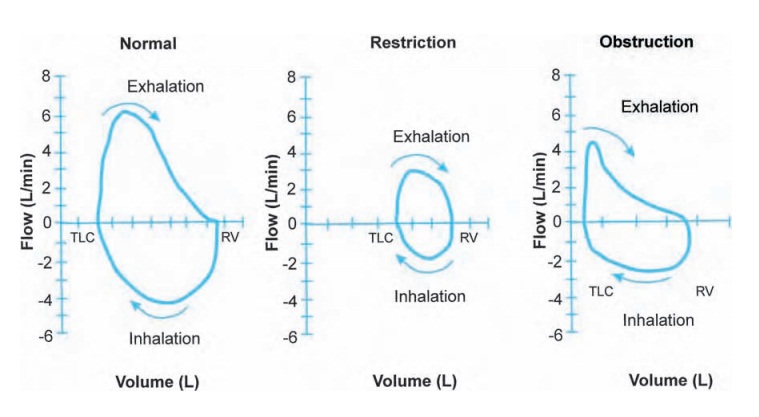
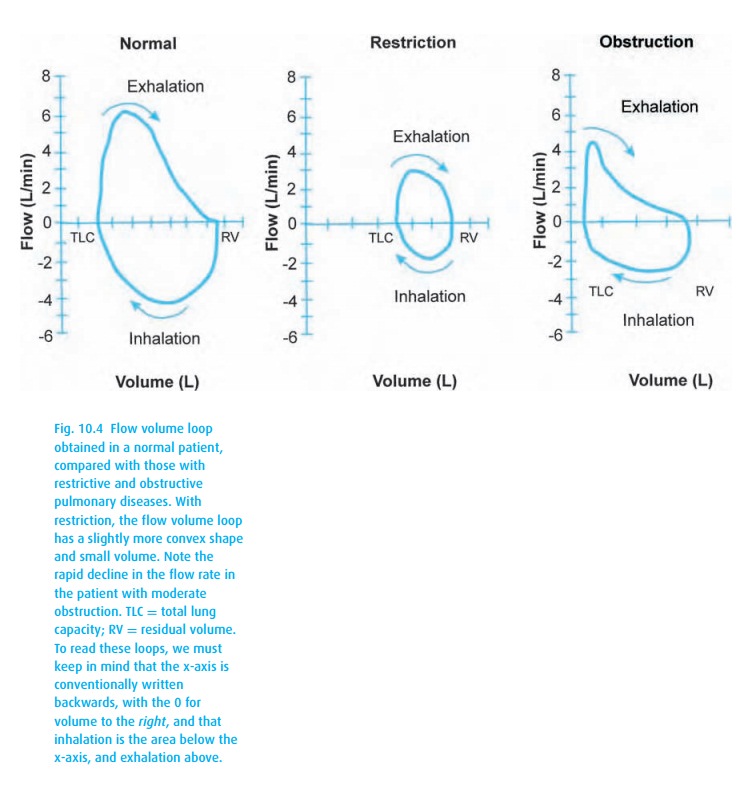
look at the percentage predicted. Flow volume loops (Fig. 10.4) can also be helpful.
Arterial blood gas analysis
When we
call for an analysis of arterial blood gases (ABG), we are really asking about
the function of two organs: lungs and kidneys. An ABG reports the partial
pressures of oxygen and carbon dioxide in arterial blood, both clearly related
to lung function, but also provides the pH and bicarbonate concentration, which
tells us something about how the kidneys are handling non-volatile acids and
bases.
In the
laboratory, the ABG values are corrected to 37 °C. This facilitates
interpret-ation of data because of the complexities introduced by temperature
changes: in addition to a direct effect on pH, both the dissociation constants
and solubility of gases are temperature dependent. For example, a PaCO2
of 40 mmHg at 37 °C, will drop to 25 mmHg when temperature falls 10 degrees.
The total carbon dioxide con-tent stays the same, but the distribution of the
components of the CO2–carbonic acid–bicarbonate complex has changed
(see below). Thus, if not corrected in the laboratory, a drop in temperature
from 37 °C to 27 °C would raise the reported pH of the blood sample from 7.4 to
about 7.54.
Oxygen
The lab
reports the partial pressure of oxygen in arterial blood as PaO2 in
mmHg, and the saturation of arterial hemoglobin with oxygen as % SaO2.
Most ABG ana-lyzers calculate the SaO2
based on a standard, adult oxyhemoglobin dissociation curve. When there is
doubt regarding the actual saturation, e.g., in the patient with suspected
carbon monoxide inhalation, we must order co-oximetry, which analyzes the
transmission of several wavelengths of light, the better to distinguish reduced
from oxygenated hemoglobin, as well as met- and carboxyhemoglobins. Please
observe the convention of writing SaO2 for the laboratory
calculation of arterial hemoglobin saturation, SpO2 for the
estimation of this value by pulse oximetry, and specify SaO2 by
co-oximetry if available. These values are rarely, if ever, identical but
usually agree within a percent or so.
Carbon dioxide
Carbon
dioxide in blood affects the pH because CO2 in an aqueous medium
(i.e. blood) will form carbonic acid, which dissociates into bicarbonate and
hydrogen ions:
CO2+ H2O ↔ H2CO3↔ H++ HCO−3
We often
describe this relationship with Henderson
Hasselbalch’s5 equation:

where pKa= the dissociation constant for carbonic acid (∼6.1), that is, the pH at which 50% of the weak
carbonic acid is ionized into equal amounts of HCO−3 and H2CO3 (for which PCO2× 0.03 is substituted). Thus the ratio
of bicarbonate to PCO2 determines pH, not their individual
concentrations.
From
this equation, we see that if we add carbon dioxide, the pH drops (res-piratory
acidosis). The interaction of CO2 with water will lead to the
generation of carbonic acid, which will lower the pH while increasing
bicarbonate, without which the pH would be even lower. Slowly (over 1–2 days),
the kidneys retain extra bicarbonate to further offset the acidosis, although
never completely correcting it. When faced with an ABG demonstrating
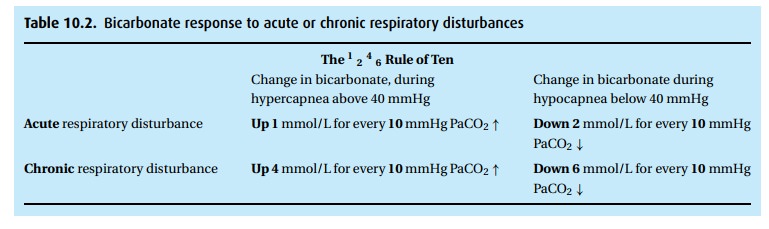
respiratory acidosis (decreased pH and increased PCO2), we can use
the 1–2–4–6 Rule of Ten (see Table 10.2),
which simply says that the indicated acute or chronic respiratory disturbance
will cause the bicarbonate to change. If that change did not take place or is
exaggerated, we need to look for metabolic explanations.
Bicarbonate
The
addition of acids, e.g., keto acids in diabetes, will also lower the pH. From
Henderson Hasselbalch’s equation, we predict that the addition of hydrogen ions
(lower pH) will cause the bicarbonate to gobble up some of the H+ (lowering the concentration of HCO−3), leading to the generation of more carbonic
acid, which can then dissociate into CO2 and water. The CO2
gas can be exhaled, thus reducing the effect of having added hydrogen ions.
Anion gap
The
addition of many acids will increase the anion gap. Recognizing there must
always be electroneutrality, if we add up all the cations (Na+ , K+ , Ca2+ , Mg2+ ), they must equal all the anions (HCO−3, Cl− , PO34− , SO34− , proteins, organic acids). Since we do not
routinely measure the proteins and organic acids, these become the “anion gap,”
the difference between cations and anions.6
We simplify all this math by only counting sodium, chloride, and bicarbonate
and accepting as normal an anion gap of 12 mEq/L +/− 4 mEq/L thus,
Anion
gap = Na+− (Cl−+ HCO−3)
Buffers
Were it
not for buffers in blood and tissue, any change in hydrogen ion concentra-tion
would cause large swings of pH. The buffers in blood (primarily hemoglobin)
avidly sop up hydrogen ions, mitigating shifts in pH; therefore, a severely
anemic patient will experience greater shifts in pH than a patient with normal
hemoglobin values. When buffering proves insufficient and correcting a low
cardiac output fails to help, we must treat a serious metabolic acidosis with
the titration of bicar-bonate. We calculate the initial dose7 as
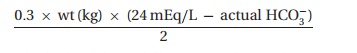
which
should not fully correct the acidemia. We then look at repeated blood gas
values presenting pH, bicarbonate, and PCO2, realizing that the
addition of bicarbonate will increase the pH while also liberating CO2,which
must (if possible) be exhaled.
ABG interpretation
A normal
room air8 arterial blood gas in a
nonpregnant9 patient should look
something like:
pH 7.35−7.45, PCO2 35−45 mmHg, PO2 75−100 mmHg,
HCO−3 22−26 mmol/L.
Other
values include methemoglobin (met Hb) <2%, carboxyhemoglobin (CO Hb) <3%, and base excess −2 to 2 mEq/L.
When the
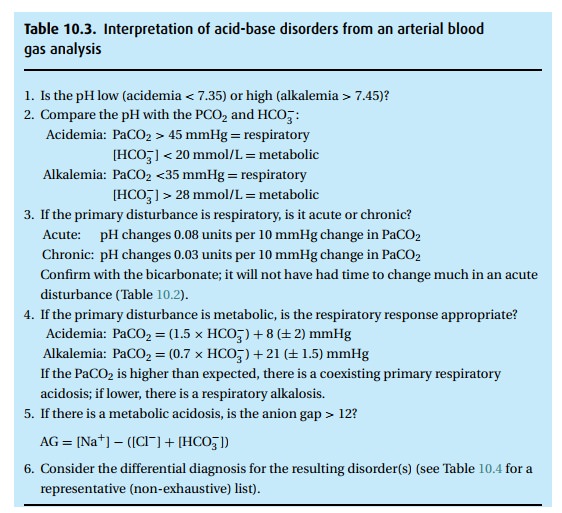
laboratory reports abnormal results, we ask several questions:
(i)Is the PO2 OK? Apply alveolar air
equation.
(ii) Is the pH OK? If not, is there a metabolic or
respiratory disturbance with or without compensation or is it a mixed
disturbance? (See Table 10.3.)
Two clinical examples
Case 1: A PACU nurse calls because a post-operative patient
has a low SpO2, whichdid not normalize by giving the patient oxygen
by face mask.
The
findings:
(i) SpO2 of 90% on FiO2 of
0.5.
The alveolar air equation estimates
(presuming – probably falsely – a normal arterial CO2 of 40 mmHg) an
alveolar oxygen tension of 306 mmHg (0.50 × (760–47) – (40/0.8)). A SpO2 of 90% corresponds to a
PaO2 of approximately 60 mmHg. Thus there is a large A-a difference.
(ii) ABG: pH 7.28, PCO2 55 mmHg, PO2
60 mmHg, HCO−3 26 mmol/L.
The
measured PaO2 corresponds (miraculously exactly) with the estimated
PaO2 using the SpO2 data. The elevated PaCO2
of 55 mmHg indicates hypoven-tilation, which would explain the low PaO2.
Step 1: The pH of 7.28 indicates acidemia.
Step 2: The elevated arterial carbon dioxide tension
implies a respiratory source.
Step 3: The CO2is increased (55 – 40=15 mmHg). The pH fall of 0.12 (7.40 – 7.28)is consistent with an acute respiratory acidosis (0.08 per 10 mmHg rise in the CO2). In confirmation, according to Table 10.2, an acute elevation of PCO2 by 15 mmHg should be associated with a rise of bicarbonate of 1.5 mEq/L.
The
patient’s bicarbonate confirms our suspicion that we are dealing with an acute,
i.e., so far uncompensated, respiratory acidemia.
We must
now determine whether obstruction (is the airway patent? Do bandages impede
ventilation?), weakness (is there a muscle relaxant hangover?), or central
depression (effects of narcotics?) can explain the hypoventilation. Therapy
will depend on what we find.
Case 2: The ambulance brings a trauma patient to the
Emergency Department.The patient has a fractured hip. As a routine, a nurse
applies a face mask deliv-ering 50% oxygen. SpO2 is 100%. An ABG
reveals: pH 7.23, PCO2 25 mmHg, PO2 250 mmHg, HCO3− 12 mmol/L.
We welcome the SpO2 of 100% but realize that the patient
can still have a ventilation/perfusion abnormality. However, the PaO2
of 250 mmHg confirms a small A-a difference (PAO2= 0.5 × (760−47) − 25/0.8 = 325 mmHg).
Step 1: The pH of 7.23 shows acidemia.
Step 2: The low PaCO2and HCO−3describe a metabolic source (with
significanthyperventilation).
Step 3: The respiratory response is appropriate (PCO2≈25≈(1.5×12)+8 mmHg).
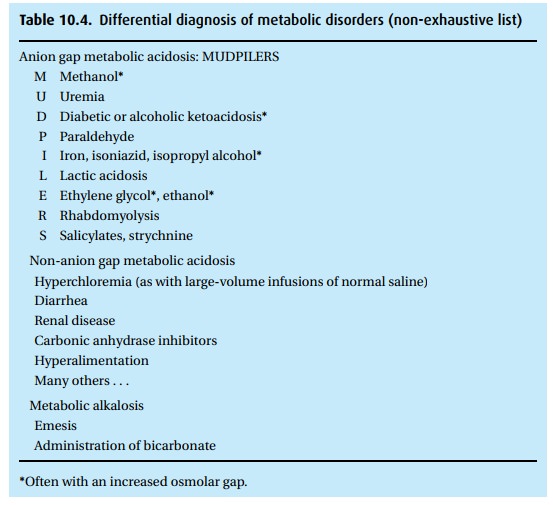
We
follow up with additional studies, such as electrolyte levels to calculate the
anion gap, to identify the cause of the patient’s trouble (Table 10.4).
Related Topics