Chapter: Biotechnology: Protein Structure And Engineering
Purification of Proteins
Purification of Proteins
Isolation of a protein from a microbial culture, plant and animal sources involves various separation techniques. These steps are collectively known as downstream processing. In spite of a large biodiversity of microbes we are restricted to certain bacteria/organisms which can be used as a source of protein as well as for introducing genes. These microorganisms are designated as "generally regarded as safe" (GRAS). GRAS listed organisms are non-pathogenic, non-toxic and generally should not produce any antibiotics. Similarily, plant tissue derived enzymes which have application in the food industry must be obtained from only non-toxic, edible plant species. One of the best known industrially useful enzymes is papain obtained from the latex of the green fruit and leaves of the Papaya tree. This enzyme finds application in meat tenderisation, clarification of beverages, digestive aids and wound cleaning solutions.
The existence of slaughter house facilities in which large number of animals are regularly processed to provide meat has also facilitated the collection of significant quantities of a particular tissue required as a protein source. Insulin is a classic example of a peptide hormone obtained from the pancreas of cows and pigs till the 1980's. Classical biotechnology required the slaughtering of over 100 pigs or 15 cows to meet the insulin requirements for one diabetic person for about one year. A rough estimate puts the number of diabetics in India by the year 2020 as 20 million! Obviously the requirement of insulin cannot be met by slaughtering pigs and cows. Fortunately, the advent of genetic engineering has ensured the availability of recombinant human insulin expressed in bacteria. Attempts are on to create transgenic animals by direct micro-injection of DNA into ova or stem cells and produce insulin and other proteins in milk on a commercial scale. This technology is called Molecular Pharming (Producing pharmaceuticals using genetically modified plants or animals). Advantages of producing recombinant proteins in milk are:
1. High production capacity.
2. Ease of source material collection (milking cows).
3. Moderate capital instrument requirements and low operational cost.
4. Ease of production including purification and scale-up.
Some medically useful peptides such as oxytocin have also been produced by direct chemical synthesis. In spite of different sources of proteins, the general principles of purification are similar.. The exact details of the purification scheme for any given protein will depend upon a number of factors such as:
1. Exact source material chosen and location of the target protein (intracellular or extracellular).
2. Quantity of protein required and hence amount of raw material processed.
3. Physical, chemical and biological properties of the protein.
Calculation of amount of bacterial ferment required
Question: An E. coli cell produces at least 2000 different proteins. One of these is our enzyme of interest produced at a level of 3000 molecules per cell under optimum conditions. If we have to purify 1g of this intra-cellular enzyme, estimate how many cells of bacteria will be required theoretically? It is given that the molecular weight of the enzyme of interest is 1,00,000.
Answer: 1,00,000 g of the protein of interest corresponds to 1 mole of enzyme which corresponds to 6.023 x 1023 molecules (Avagadro no.)
Hence 1 g of enzyme has 1/1,00,000 x 6.023 x 1023 or 6.023 x 1018 molecules .
3000 molecules of the enzyme are present in one cell.
Therefore, 6.023 x 1018 molecules are present in 6.023 x 1018/3000 = 2.007 x 1015 cells.
Question: Assuming that the bacterial cell is a cylinder (d = 1µm, h= 2µm) calculate (a) the total packed cell volume of E.coli required to produce 1 g of intra-cellular enzyme (b) the volume of the fermentor required if the maximum cell concentration inside the fermentor is 5% (cells need space to multiply).
Answer: Volume of a single bacterium = ðr2h (cylinder volume) = 3.142 x 0.5 x 0.5 x10-12 x2 x10-6
(note 1µm = 10-6m, r = ½d) =1.57 x 10-18 m3
2.007 x1015 cells (see previous answer) would have a volume of 2.007 x 1015 x 1.57 x 10-18 m3
= 3.15 x 10-3 m3 = 3.15 L Answer (a)
(1L = 10-3 m3)
Answer (b) 100% concentration = 3.15 L
Therefore 5% concentration = 100/5 x 3.15 = 63 L
Volume of the fermentor required would be more than 63 L (30% extra space) about 82 L.
The source material chosen will dictate the range and type of contaminants present in the starting material. If the protein is extracellular, then one needs to separate the cellular components and process the medium to isolate the protein of interest. However if the protein is intracellular then the choice of method of cell disruption will depend on the cell type. Plant and fungal cells require harsher breakage methods; animal cells are easier to break because of no cell wall. Bacterial cells being very small require high pressure techniques. Once the proteins are wall. Bacterial cells being very small require high pressure techniques. Once the proteins are released into suitable buffered solutions a variety of physico-chemical techniques are applied to selectively purify the protein of interest from the others.
Genetically engineered proteins are often tagged with certain molecules in order to confer some very pronounced physico-chemical characteristics on the protein of interest. This renders its separation from contaminants more straightforward. The ability to detect and quantify the total protein levels is an essential pre-requisite to the purification and characterisation of any protein. A typical purification scheme can be analysed as follows (Table 1).
Table 1. Typical purification table
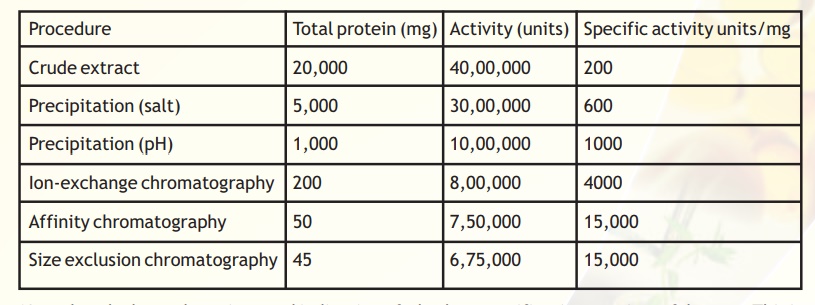
Note that the last column is a good indication of whether a purification step is useful or not. This is because as a protein is purified its specific activity increases because the denominator should ideally decrease as irrelevant proteins are removed and only the specific protein/enzyme of interest is concentrated. Hence from the given table it is apparent that the step following affinity chromatography, size exclusion chromatography, is redundant as the specific activity does not change. For activity measurements it is also important to choose a proper assay method reflecting the sensitivity required and further the method should be specific. In the case of proteins absorbance measurements at 280 nm is easy, fast and non-destructive procedure for monitoring the concentration.
Bioassays can sometimes be more sensitive than chemical assays. However one needs suitable standards of known bioactivity values to arrive at a correct activity of the unknown sample. Where a protein of biological interest is concerned, example insulin, bioassays are mandatory. If the sample particularily has to be injected other safety tests such as toxicity have to be performed.
Downstream Processing
After cells (bacterial, animal or plant) have grown to their requisite capacity in a fermentor it becomes necessary to harvest the cells or medium depending in which component the recombinant protein is expressed and then purify the protein from other substances. These processes are part of downstream processing and because of the large amounts of source (a fermentor can be more than 1000 L capacity) bulk separation methods are used which are different from laboratory scale purification although principles involved are similar.
In the case of intracellular microbial proteins, cell harvesting is done by filtration or centrifugation from the fermentation medium, followed by re-suspension of cells in buffer or water with subsequent cell disruption. Most proteins obtained from plant and animal tissues are intracellular in nature. The initial step involves collection of the appropriate tissue, for example collection of blood to obtain proteins, collection of pituitary glands to obtain pituitary hormones etc.
Aqueous two-phase partition
When a crude cell homogenate is added to a biphasic mixture of dextran and polyethylene glycol (PEG) the cellular debris partitions to the lower, more polar and dense phase, dextran. Separation of the two phases achieves effective separation of cellular debris from soluble protein (Fig. 8).
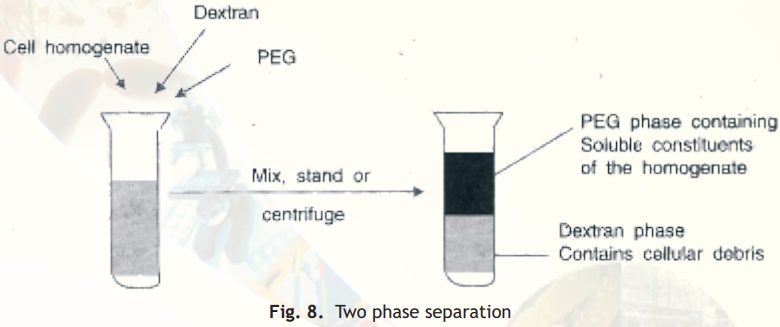
Fig. 8. Two phase separation
In some cases it is desirable and necessary to remove or destroy the lipids and nucleic acid of a cell homogenate as it may be a contaminant and can interfere with subsequent purification steps. The lipid layer can be removed by passage of solution through glass wool or cloth of very fine mesh size. Effective removal of nucleic acids may be achieved by precipitation or by treatment with nucleases.
For large scale application, concentration of extracts is normally achieved by precipitations, ion-exchange chromatography or ultrafiltration.
At any given pH value proteins display either a net positive, negative or no charge. Using these parameters different protein molecules can be separated from one another by judicious choice of pH, ionic strength and ion-exchange materials.
All efforts should be made to maximise protein stability during various steps. Some of the general conditions which may be followed are:
1. Maintenance of a specific pH value range of buffered solutions in which a protein is maximally stable.
2. Maintenance of physiological conditions (%CO2 for animal cell culture and temperature).
3. Use of inhibitors to prevent the action of proteolytic enzymes.
4. Avoidance of agitation or addition of chemicals which may denature the target protein.
5. Minimise processing time.
Industrial scale production of proteins
The laboratory scale design cannot be scaled up to industrial scale directly. The following points need attention for industrial scale production:
1. Bulk purchase of chemicals and other raw materials would bring down costs.
2. The labour cost decreases sharply with increase in production.
Most large scale process equipment such as holding vessels and transfer pumps are constructed from stainless steel or plastics, such as polypropylene. Glass vessels, so commonly used in laboratory scale culturing techniques are seldom used for large scale preparatory work. Materials used for large scale culturing must be inert and resistant to the corrosive action of any chemical used during the process. They should not allow any leaching of potentially toxic metals or chemicals into the product stream. It is useful to remember that any commercial plant has to have good GMP (good manufacturing practice) . Any downstream processing requires the approval of a regulatory authority in the form of a license to produce and market proteins designed for use in the food or health care industry. This ensures that the processing procedures are based upon established, validated methodologies. Generally 80% of the overall production cost are due to steps in downstream processing and quality assurance.
A generalised downstream processing scheme used in the production of bulk protein/enzyme from microbial sources is given in Fig. 9. Similar steps can be applied for animal and plant sources.
Special techniques for therapeutic /diagnostic proteins
These proteins must be purified to a very high degree especially for use in parenteral (injectable) administration. They also have to be sterile products (free of bacterial and fungal contamination) and hence can be administered by injection, infusion or implantation.
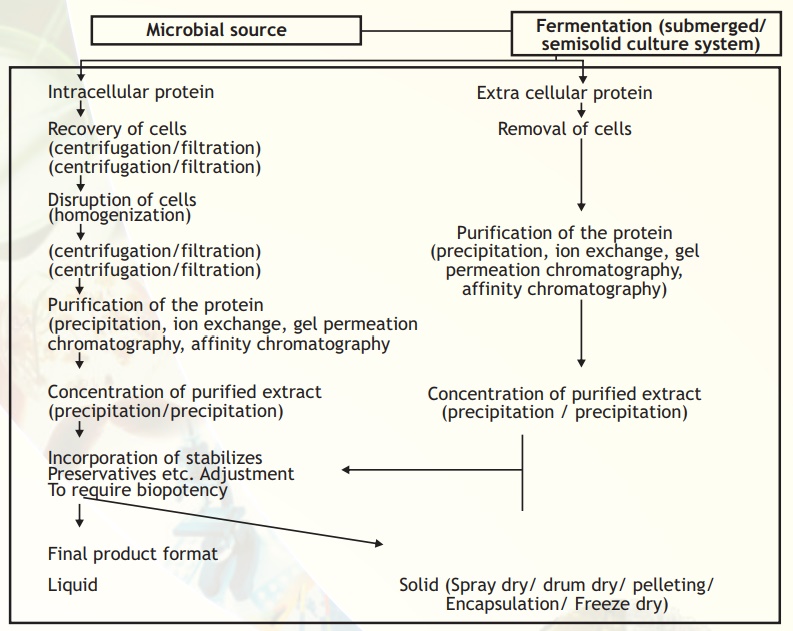
Fig. 9. A typical flow sheet from source to product.
Related Topics