Chapter: Basic & Clinical Pharmacology : Heavy Metal Intoxication & Chelators
Pharmacokinetics, Pharmacodynamics - Toxicology of Arsenic
Pharmacokinetics
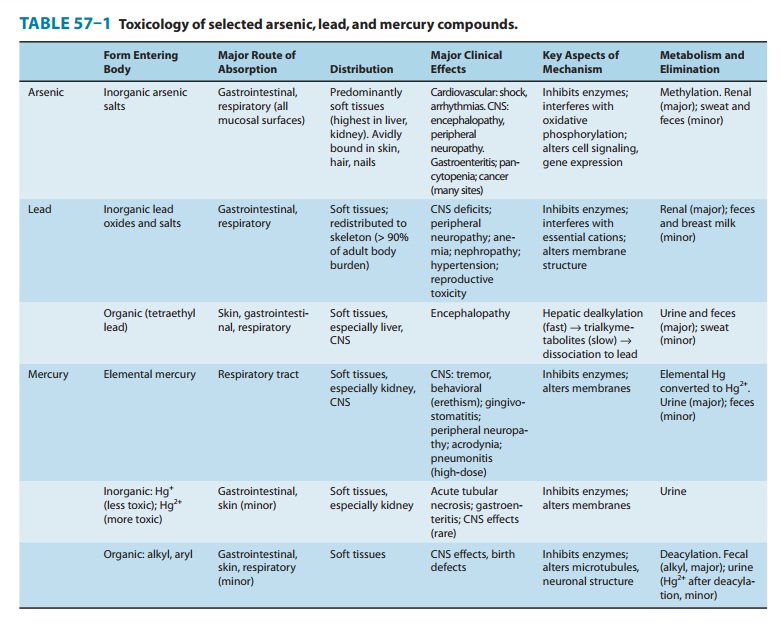
Soluble arsenic compounds are well absorbed through the respira-tory and gastrointestinal tracts (Table 57–1). Percutaneous absorp-tion is limited but may be clinically significant after heavy exposure to concentrated arsenic reagents. Most of the absorbed inorganic arsenic undergoes methylation, mainly in the liver, to monomethyl-arsonic acid and dimethylarsinic acid, which are excreted, along with residual inorganic arsenic, in the urine. When chronic daily absorption is less than 1000 mcg of soluble inorganic arsenic, approximately two thirds of the absorbed dose is excreted in the urine within 2–3 days. After massive ingestions, the elimination half-life is prolonged. Inhalation of arsenic compounds of low solu-bility may result in prolonged retention in the lung and may not be reflected by urinary arsenic excretion. Arsenic binds to sulfhy-dryl groups present in keratinized tissue, and following cessation of exposure, hair, nails, and skin may contain elevated levels after urine values have returned to normal. However, arsenic in hair and nails as a result of external deposition may be indistinguishable from that incorporated after internal absorption.
Pharmacodynamics
Arsenic compounds are thought to exert their toxic effects by several modes of action. Interference with enzyme function may result from sulfhydryl group binding by trivalent arsenic or by substitution for phosphate. Inorganic arsenic or its metabolites may induce oxidative stress, alter gene expression, and interfere with cell signal transduction. Although on a molar basis, inorganic trivalent arsenic (As3+, arsenite) is generally two to ten times more acutely toxic than inorganic pentavalent arsenic (As5+, arsenate), in vivo interconversion is known to occur, and the full spectrum of arsenic toxicity has occurred after sufficient exposure to either form. Recent studies suggest that the trivalent form of the methy-lated metabolites (eg, monomethylarsonous acid [MMA III])may be more toxic than the inorganic parent compounds. Reduced efficiency in the methylation of MMA to DMA, result-ing in an elevated percentage of MMA in the urine, has been associated with an increased risk of chronic adverse effects. Arsenic methylation requires S-adenosylmethionine, a universal methyl donor in the body, and arsenic-associated perturbations in one-carbon metabolism may underlie some arsenic-induced epigenetic effects such as altered gene expression.
Arsine gas is oxidized in vivo and exerts a potent hemolytic effect associated with alteration of ion flux across the erythrocyte membrane; it also disrupts cellular respiration in other tissues. Arsenic is a recognized human carcinogen and has been associated with cancer of the lung, skin, and bladder. Marine organisms may contain large amounts of a well-absorbed trimethylated organo-arsenic, arsenobetaine, as well as a variety of arsenosugars and arsenolipids. Arsenobetaine exerts no known toxic effects when ingested by mammals and is excreted in the urine unchanged; arsenosugars are partially metabolized to dimethylarsinic acid. Thio-dimethylarsinic acid has recently been identified as a com-mon but minor human arsenic metabolite of uncertain toxico-logical significance.
Related Topics