Chapter: Biotechnology Applying the Genetic Revolution: Recombinant Proteins
Increasing Protein Stability
INCREASING
PROTEIN STABILITY
Different proteins vary
greatly in stability. The lifetime of a protein inside a cell depends mainly on
how fast the protein is degraded by proteolytic enzymes or proteases. Apart
from the overall 3D structure of the protein, there are two specific factors
that greatly affect the rate of protein degradation. These are believed to act
by altering the recognition of proteins by the degradation machinery.
(a)
The identity of the N-terminal amino acid greatly affects the
half-life of proteins. Although all polypeptide chains are made with methionine
as the initial amino acid, many proteins are processed later. Therefore the
N-terminal amino acid of mature proteins varies greatly. The N-terminal rule
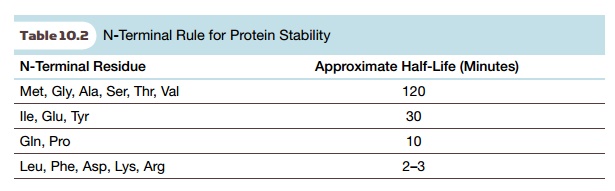
for stability is shown in Table 10.2.
(b)
The presence of certain internal sequences greatly destabilizes
proteins. PEST sequences are regions of 10 to 60 amino acids that are rich in P
(proline), E (glutamate), S
(serine), and T (threonine). The PEST sequences create domains whose structures
are recognized by proteolytic enzymes.
Alteration of the N-terminal
sequence of a protein by genetic engineering is relatively simple. A small
segment of artificially synthesized DNA encoding a new N-terminal region can be
incorporated by standard methods, e.g., PCR (see Chapter 4). The effectiveness
of this approach has been demonstrated experimentally for β-galactosidase. Changing an internal PEST
sequence without disrupting protein function is a vastly more complex
undertaking, even though in theory it could lead to greater stability and
therefore greater yields.
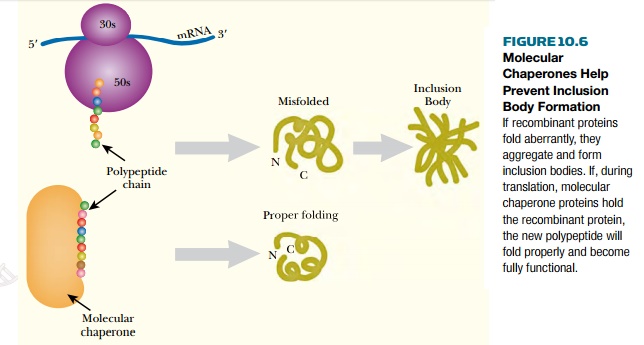
In some instances, proteins, which would be stable if folded correctly, fold aberrantly during synthesis. The misfolded proteins form inclusion bodies, which are dense particles visible with optical microscopy. Inclusion bodies are usually formed when recombinant proteins are poorly soluble or produced too fast. One method to alleviate the aggregation is to provide molecular chaperones that help fold the recombinant protein correctly. Molecular chaperones attach themselves to polypeptides while they are being translated and help keep the protein unfolded until translation is complete. Then the protein can fold into its correct shape (Fig. 10.6).
Related Topics