Chapter: Biotechnology Applying the Genetic Revolution: Recombinant Proteins
Expression of Proteins by Mammalian Cells
EXPRESSION OF PROTEINS BY
MAMMALIAN CELLS
Some cloned animal genes may ultimately need to be expressed in cultured mammalian cells. To facilitate this, mammalian shuttle vectors exist that allow movement of a cloned gene from bacteria to mammalian cells (Fig. 10.13). As usual, such vectors contain a bacterial origin of replication and an antibiotic resistance gene allowing selection in bacteria. These vectors must also possess an origin of replication that works in mammalian cells. Usually this is taken from a virus that infects animal cells, such as SV40 (simian virus 40). Viral promoters are often used because they are strong, producing copious amounts of protein. Alternatively, promoters from mammalian genes that are expressed at high levels (e.g., the genes for metallothionein, somatotropin, or actin) may be used. The multiple cloning sites lie downstream of the strong promoter. Since animal genes are normally cloned as the cDNA, the vector must also provide a polyadenylation signal (i.e., tail signal) at the 3’-end of the inserted gene.
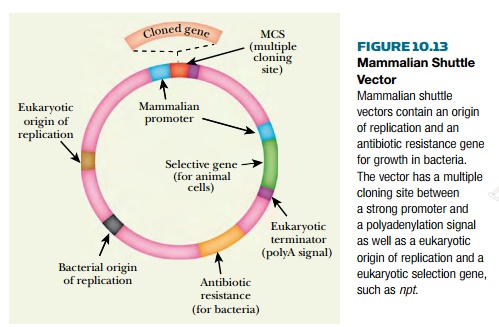
Several selective markers may
be used with animal cells. Very few antibiotics kill animal cells. However, the
antibiotic genetecin, also known as G-418, kills animal cells by blocking
protein synthesis. G-418 is related to antibiotics such as neomycin and
kanamycin that are used against bacteria. The npt (NeoR) gene, encoding neomycin phosphotransferase, adds a
phosphate group to neomycin, kanamycin, and G-418, which inactivates these
antibiotics. Consequently, npt can be
used to select animal cells using G-418 or bacterial cells using neomycin or
kanamycin.
Because of the lack of
antibiotics for mammalian cells, mutant eukaryotic cells lacking a particular
enzyme are sometimes used for selection. The plasmid then carries a functional
copy of the missing gene. This rather inconvenient approach has been used in
both yeast and animal cells. In yeast, genes for amino acid biosynthesis have
been used. In animal cells, the DHFR gene, encoding dihydrofolate
reductase, is sometimes used. This enzyme is required for synthesis of the
essential cofactor folic acid and is inhibited by methotrexate. Mammalian cells lacking the DHFR gene are used, and a functional copy of the DHFR gene is provided on the shuttle
vector. Treatment with methotrexate inhibits DHFR and hence selects for
high-level expression of the DHFR gene on the vector. Methotrexate levels can
be gradually increased, which selects for a corresponding increase in copy
number of the vector. (The chromosomal DHFR
genes must be absent to avoid selecting chromosomal duplications rather than
the vector.)
An alternative approach is to use a metabolic gene as a dominant selective marker. The enzyme glutamine synthetase protects cells against the toxic analog, methionine sulfoximine. The resistance level depends on the copy number of the glutamine synthetase gene. Therefore, multicopy plasmids carrying the glutamine synthetase gene can be selected even in cells with functional chromosomal copies of this gene.
Related Topics