Chapter: Biotechnology Applying the Genetic Revolution: Recombinant Proteins
Expression of Proteins by Eukaryotic Cells
EXPRESSION OF PROTEINS BY EUKARYOTIC CELLS
Although bacterial cells have
successfully expressed many eukaryotic proteins, there are cases where it is
best to express eukaryotic proteins using eukaryotic cells. Some eukaryotic
proteins are unstable or inactive after being made by bacterial cells. This is
especially true of proteins that require posttranslational modification.
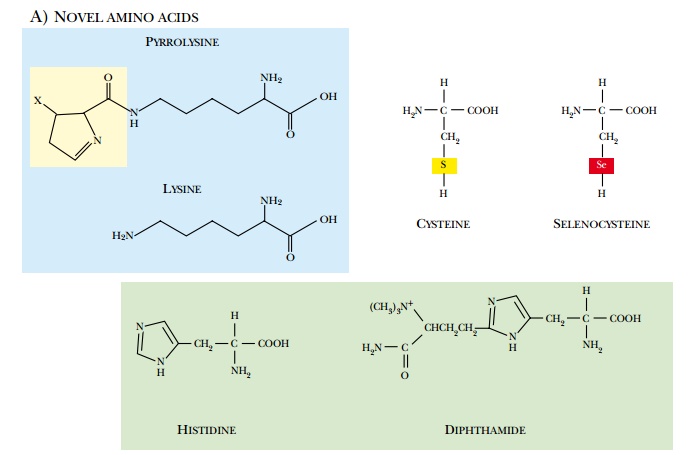
A variety of eukaryotic
modifications may occur after the polypeptide chain has been made (Fig. 10.8).
These include:
(a)
Chemical modifications that form novel amino acids in the
polypeptide chain.
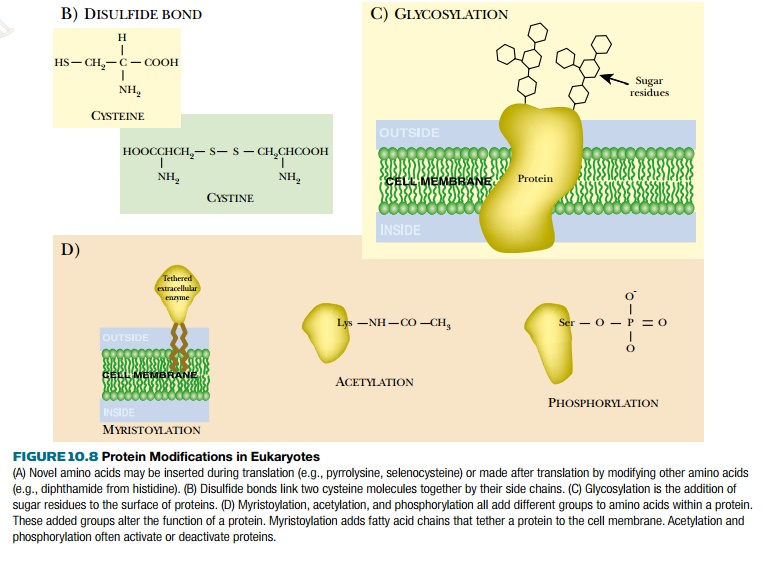
(b)
Formation of disulfide bonds between correct cysteine partners
(e.g., the assembly of insulin).
(c)
Glycosylation, that is, the addition of sugar residues at specific
locations on the protein. Many cell surface proteins are glycosylated and will
not assemble correctly into membranes or function properly if lacking their
glycosyl components.
(d)
Addition of a variety of extra groups, such as fatty acid chains,
acetyl groups, phosphate groups, sulfate groups.
(e)
Cleavage of precursor proteins. This may occur in several stages,
as illustrated by insulin. Cleavage may be involved with secretion, correct
folding, and/or activation of proteins.
The enzymes required for modification
and processing are normally absent from bacterial cells, making it necessary to
express eukaryotic proteins in eukaryotic cells. Related processing enzymes are
often present in a range of higher organisms; thus it is rarely absolutely
necessary to express a protein in its original organism. Here we are concerned
with protein production in cultured cells. However, as discussed, it is now
possible to engineer whole transgenic animals or plants to produce recombinant
proteins. A further advantage of expressing eukaryotic proteins in eukaryotic
cells is that contamination with bacterial components is avoided. Despite
purification, bacterial components that are toxic or promote immune reactions
or cause fever may be present in traces if bacteria are used for production.
As discussed in Previews Pages, shuttle vectors
are designed to move genes between different groups of organisms. Because
genetic engineering is more difficult for eukaryotes, most expression vectors
for eukaryotic cells are in fact shuttle vectors. Such vectors allow genetic
engineering to be carried out in bacteria, usually E. coli, and allow transfer to other organisms for gene expression.
We will consider the use of yeasts, insect cells, and mammalian cells for
expression of recombinant proteins.
Related Topics