Chapter: Organic Chemistry: Alkyl halides
Alkyl halides: Organometallic reactions
ORGANOMETALLIC REACTIONS
Key Notes
Grignard reagents
Alkyl
halides are converted to Grignard reagents by treating them with magnesium
metal in dry ether. The magnesium is bonded between the car-bon and the halogen
of the original C–X bond and converts the carbon from an electrophilic center
to a nucleophilic center. Grignard reagents react as if they were the
equivalent of carbanions. Grignard reagents are particularly useful in the
synthesis of larger carbon skeletons and are used in the synthesis of alcohols,
ketones, and carboxylic acids.
Organolithium reagents
Alkyl
halides are converted to organolithium reagents by treatment with lithium metal
in an alkane solvent. They react similarly to Grignard reagents.
Organocuprate reagents
Alkyl
halides can be converted to organolithium reagents which can then be converted
to organocuprate reagents. These reagents are useful in conjugate additions to α,β-unsaturated carbonyls and for linking alkyl
halides.
Grignard reagents
Alkyl halides of all types (1°, 2° and 3° ) react with magnesium in dry ether to form
Grignard reagents, where the magnesium is ‘inserted’ between the halogen and
the alkyl chain (Fig. 1).
These reagents are extremely useful in organic synthesis and can be used in a wide variety of reactions. Their reactivity reflects the polarity of the atoms pre- sent. Since magnesium is a metal it is electropositive, which means that the elec- trons in the C–Mg bond spend more of their time closer to the carbon making it slightly negative and a nucleophilic center. This reverses the character of this carbon since it is an electrophilic center in the original alkyl halide. In essence, a Grignard reagent can be viewed as providing the equivalent of a carbanion. The carbanion is not a distinct species, but the reactions which take place can be explained as if it was.
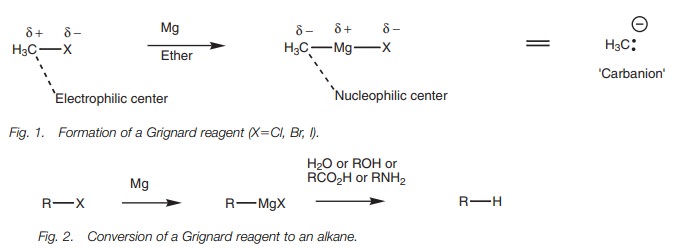
A Grignard reagent can react as a base with
water to form an alkane. This is one way of converting an alkyl halide to an
alkane. The same acid–base reaction can take place with a variety of proton
donors (Brønsted acids) including functional groups such as alcohols,
carboxylic acids, and amines (Fig. 2).
This can prove a disadvantage if the Grignard reagent is intended to react at
some other site on the target molecule. In such cases, functional groups
containing an X–H bond (where X a heteroatom) would have to be protected before
the Grignard reaction is carried out.
The reactions of Grignard reagents have been
covered elsewhere and include reaction with aldehydes and ketones to give
alcohols, reaction with acid chlorides and esters to give tertiary alcohols ,
reaction with carbon dioxide to give carboxylic acids, reaction with nitriles
to give ketones , and reaction with epoxides to give alcohols.
Organolithium reagents
Alkyl halides can be converted to organolithium
reagents using lithium metal in an
alkane solvent (Fig. 3).
Organolithium reagents react similarly to Grignard reagents. For example, reaction with aldehydes and ketones results in nucleophilic addition to give secondary and tertiary alcohols respectively .
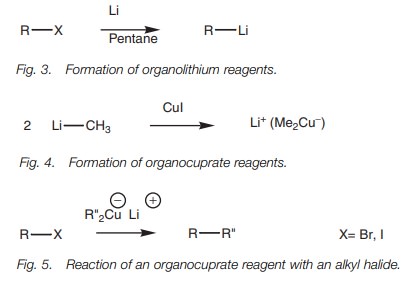
Organocuprate reagents
Organocuprate reagents (another source of
carbanion equivalents) are prepared by the reaction of one equivalent of
cuprous iodide with two equivalents of an organolithium reagent (Fig. 4).
These reagents are useful in the 1,4-addition
of alkyl groups to α,β-unsaturated carbonyl systems and can also be
reacted with alkyl halides to produce larger alkanes (Fig. 5). The mechanism is thought to be radical based.
Related Topics