Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Gene Therapy
Adenoviral Vectors - Viral Vectors for Gene Transfer
Viral Vectors for Gene Transfer
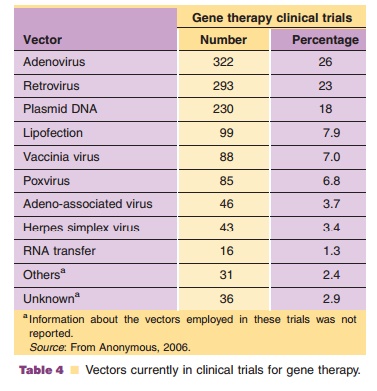
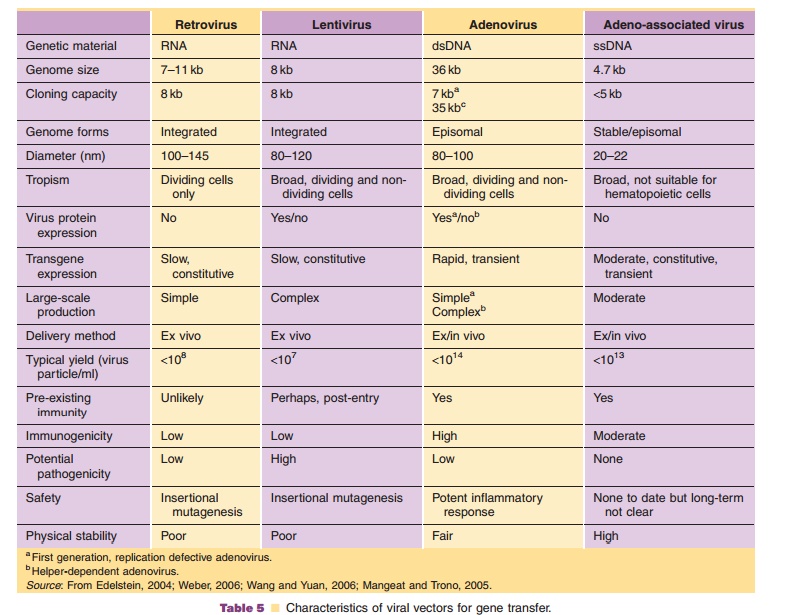
Viruses, natural parasites that efficiently enter cellular targets and hijack cellular machinery for propagation, are currently the most effective vectors for gene therapy. Approximately 70% of all gene therapy clinical trials employ viral vectors (Table 4). Many viruses with divergent properties have been devel-oped for gene transfer. Retroviruses, adenoviruses and adeno-associated viruses (AAVs) are the most extensively studied to date. Characteristics of each are summarized in Table 5.
Adenoviral Vectors
Biology
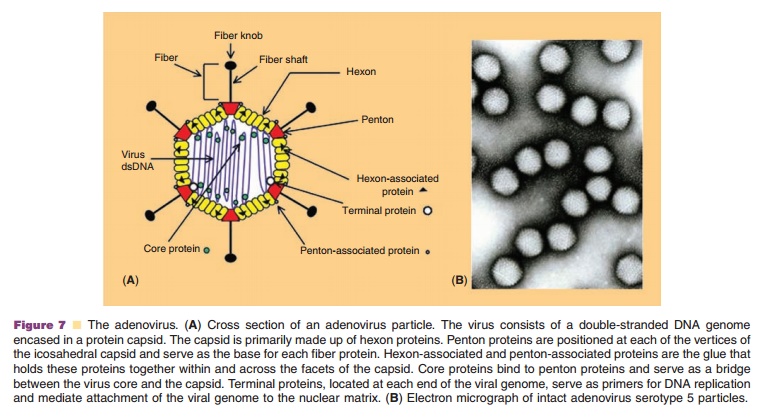
Adenoviruses are non-enveloped, lytic, DNA viruses with a linear double-stranded genome and icosahedral symmetry. Since the isolation of the first human adenovirus in 1953 from tonsils and adenoid tissue (Rowe, 1953), 50 additional serotypes have been identified and grouped into six species (A–F) based on genome size, composition and homology, hemag-glutinating properties and oncogenicity in rodents. Because the subgroup C adenoviruses 2 and 5 have been studied extensively, they were the first to be adapted as vectors for gene therapy. The virus genome, 36 kb in length, is divided into early and late transcription units, based upon the kinetics of viral infection. There are five early (E1A, E1B, E2, E3, and E4) and four intermediate (IVa2, IX, VAI, and VAII)transcriptional units (Nevins, 1987). The E1A tran-scription unit activates other early transcription units, optimizing the environment for virus replication by inducing the cell to enter the S phase of division (Berk, 2005). E1B proteins prevent apoptosis and promote cell survival. E2A encodes for DNA polymerase, pre-terminal and single-stranded DNA binding proteins, involved in virus replication (de Jong, 2003). E3 is responsible for elusion of the host immune response and promoting the survival of infected cells while products of the E4 transcription unit influence the cell cycle and cell transformation (Lichtenstein, 2004; Weitzman, 2005). Late region genes are transcribed as one long precursor transcript from the major late promoter and processed into five mRNA molecules (L1–L5) that encode capsid structural proteins (McConnell, 2004). The essential E1 region of viral genome was deleted in the first adenoviruses devel-oped for gene therapy applications to prevent replica-tion. The E3 region was also often removed to accommodate transgene expression cassettes.
Adenovirus particles are approximately 70 to 90 nm in size (Fig. 7B). The protein capsid consists of 252 protein subunits. There are 240 copies of the trimeric hexon protein (Fig. 7A). Five copies of the penton protein are located at each of the 12 vertices of the capsid and are responsible for holding fiber proteins in place. The twelve fiber proteins are asymmetrical and consist of a thin shaft protein topped by a globular knob. While it is ultimately the elements of the viral genome and the capacity of the cell to support virus infection that determine the efficiency of transgene expression, adenoviral capsid proteins also facilitate binding, entry and translocation of the genome into the cell (Medina-Kauwe, 2003).
Adenovirus infection begins with binding of the knob domain of the fiber capsid protein to the Coxsackievirus and Adenovirus receptor (CAR) (Fig. 8) (Bergelson, 1997). Adenovirus also can bind to the a2 domain of the human major histocompat-ibility complex class I (MHC I) molecule and heparan sulfate glycosaminoglycans (HS GAGs) to initiate infection (Hong, 1997; Dechecchi, 2001). After initial binding, an Arg-Gly-Asp (RGD) motif within the penton base interacts with aVb3 and aVb5 integrin receptors (Nemerow, 1999). Engagement of integrins initiates a series of cell signaling processes associated with virus infection and facilitates effi-cient internalization of adenovirus particles, escape from the endosomal compartment and nuclear targeting of the adenovirus genome (Medina-Kauwe, 2003). Adenovirus particles enter the nucleus as early as 30 minutes after initial cellular contact. Early mRNA and viral proteins can be detected within an hour after the virus binds to cellular receptors. Viral DNA replication and particle
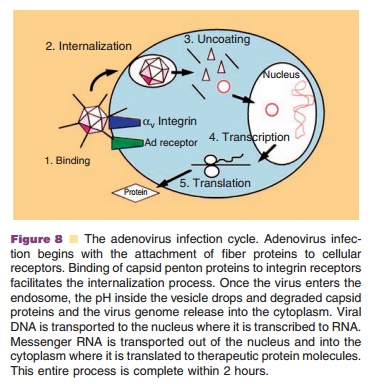
assembly in the nucleus starts 8 hours after infection and culminates in the release of 104 to 105 mature virus particles per cell 30–40 hours post-infection by cell lysis (Majhen, 2006).
Suitability of Adenoviruses for Gene Transfer
Adenoviruses have several characteristics that make them attractive for gene transfer (Table 5). Biology of the virus is well understood, making adenovirus-based vectors relatively easy to construct. High quality, purified preparations can be easily produced at titers of 1 1013 virus particles/ml in well-defined cell systems. Transgene expression from these vectors is rapid and robust and is enhanced with strong heterologous promoters. Unlike some viruses, adeno-viruses are physically stable, allowing them to survive stringent purification protocols and long-term storage. Adenoviruses can infect a wide range of dividing and non-dividing cells. Use of animal-specific adenovirus serotypes for human gene trans-fer and retargeting strategies at the molecular and macromolecular levels are under development to limit transduction to target tissues (Mizuguchi, 2004; Bangari, 2006). Adenoviruses do not integrate into the host genome. While this minimizes the risk of insertional mutagenesis, gene expression is transient, making them unsuitable for long-term correction of genetic defects. Because the adenovirus genome can accommodate large transgene cassettes, hybrid ade-novirus–adeno-associated virus vectors have been designed for region-specific chromosomal integration and prolonged transgene expression (Lundstrom, 2004).
A significant drawback to the use of recombinant adenoviruses is the ability of the virus to elicit a strong immune response. The host response to adenovirus has been well documented and occurs in three phases in animal models and humans (Nazir, 2005). The first phase occurs within an hour after systemic adminis-tration, lasts for 4 days and is characterized by thrombocytopenia and elevated liver enzymes, theseverity of which is dose dependent. The second phase, occurring 5 to 7 days after administration, is high-lighted by removal of transduced cells by activated lymphocytes in the target tissue and localized, self-limited inflammation (Muruve, 2004). During the third phase, CD4þ T-cell-dependent humoral immunity develops and neutralizing antibodies clear the virus from the circulation and prevent effective readminis-tration (Xu, 2005). Successful readministration of adenoviral vectors is difficult since ~50% to 90% of the world’s population has high levels of anti-adeno-virus serotype 5 antibodies (Nwanegbo, 2004) which have been shown to significantly reduce the efficacy of these vectors in mice, primates and humans in a phase I clinical trial (Ertl, 2005; Kresge, 2005).
The significance of the immune response gener-ated by adenoviral vectors has been illustrated in the clinic. Intra-hepatic administration of an E1/E4 deleted adenovirus failed to produce a therapeutic effect in patients enrolled in a clinical trial designed to correct ornithine transcarbamylase (OTC) deficiency (Raper, 2002). This was associated with a mild immune response to the virus and rapid clearance of vector and transgene. One patient in the highest dose cohort (6x1011 virus particles/kg) experienced a severe immune response syndrome characterized by multiorgan failure and sepsis and died soon aftertreatment (Raper, 2003). This was the first reported death ascribed to gene therapy (Raper, 2002). Likewise, a trial for hemophilia A using intravascular delivery of an adenoviral vector was stopped because of transient thrombocytopenia and transaminase elevation in patients (High, 2003). As a result, it is now recognized that recombinant adenoviruses can be safely administered directly to target organs at doses of up to 1x1011virus particles (O’Connor, 2006).
Averting the Immune Response Against Adenovirus
A significant effort has been put forth to address the problem of the adenovirus-mediated immune response. Coadministration of immunosuppressive agents such as cyclophosphamide, FK506, and cyclos-porin A extended the duration of transgene expression, but did not prevent development of neutralizing antibodies (Xu, 2005). Virus-induced toxicity was also reduced through modification of the viral genome. Second generation vectors, attenuated in regions E1–E4, have reduced the inflammatory response and extended the duration of transgene expression (Fig. 9) (McConnell, 2004). Deletion of all early and late genes in the helper-dependent adenovirus, has improved toxicity and transgene expression. Neutralizing anti-bodies, however, are still detected after treatment with any of these vectors.
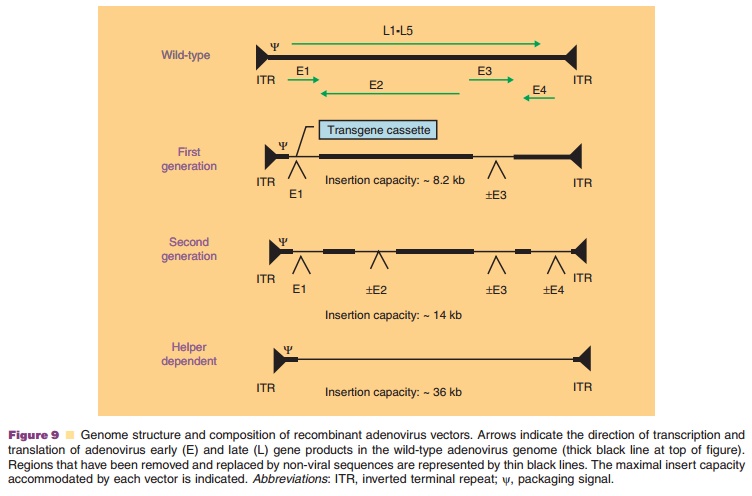
“Seroswitching,” the use of either non-human or rare human adenovirus serotypes, is one approach to address the problem of pre-exiting immunity to adenovirus serotype 5 (Bangari, 2006). Recombinant adenoviruses derived from human serotypes 35 and 11 with low exposure rates in the general population can induce high levels of transgene expression and are minimally affected by pre-existing immunity to adenovirus 5. Since antibodies capable of neutralizing virus particles are directed primarily against hexon proteins (Sumida, 2005), hexon-chimeric viruses have also been developed (Roberts, 2006). Although they avoid neutralization, they are very difficult to pro-duce. Covalent attachment of polyethylene glycol or incorporation into polymer matricies has also shown promise in protecting adenoviral vectors from neutralization (Bangari, 2006).
Clinical Use of Adenoviral Vectors
Exploitation of adenoviruses in medicine started in the late 1950s. Adenovirus serotypes 4 and 7 were first used as an oral vaccine against acute respiratory disease in United States military personnel (Top, 1971). Use of this vaccine for 25 years without significant side effects served as the platform for further development of adenovirus-based vectors for gene transfer. Today, approximately 26% of all gene therapy clinical trials involve recombinant adenoviruses, making them the most widely used vector for gene transfer (Table 4). There are currently 16 trials employing adenovirus-based vectors in the phase II/III stage of clinical testing that focus on treatments for cancer and cardiovascular disease. In 2003, Gencidine (SiBiono, Shenzen, China), a replica-tion deficient adenovirus serotype 5 vector expressing the p53 transgene, was the first gene therapy treat-ment for cancer to be approved by China’s SFDA for worldwide clinical use in head and neck squamous cell carcinoma (Peng, 2005). It and a similar product (INGN 201, Advexin, not yet approved for wide-spread use (Gabrilovich, 2006)) are currently being tested for treatment of glioma, non-small cell lung cancer, bladder carcinoma and ovarian cancer alone or in combination with chemotherapy and radiation. Because adenoviruses can quickly induce a strong immune response, they can naturally serve as a powerful adjuvant to promote a strong immune response against an encoded antigen for vaccination purposes. Virus encoded with sequences of antigens derived from pathogens such as anthrax, plague, hepatitis C, rabies and SARS successfully induced strong immune responses often correlating with protection of susceptible animals against challenge with the infectious agent (Boyer, 2005; Zhou, 2006). Many of these vectors will soon be tested in clinical vaccination protocols.
Production and Processing of Recombinant Adenoviruses
Traditionally, recombinant adenoviruses are gener-ated by transfecting E1-complementing human cell lines such as 293, 911, PER.C6 or N52.E6 with: (a) a shuttle plasmid containing the left adenovirus ITR and packaging domain followed by the transgene cassette in the E1 region and (b) a template consisting of most of the adenovirus genome including the right ITR and a stretch of DNA reiterated in the shuttle plasmid. Homologous recombination through the sequences shared by the two DNA molecules creates the full E1-deleted adenovirus genome. Alternative systems for recombination of the plasmids in yeast and bacteria instead of mammalian cells have simplified and accelerated this process. After assem-bly of the recombinant genome, replication and packaging, adenoviral vectors can easily be produced in large amounts in E1-complementing cells. A significant problem often encountered in 293 cells is the production of replication competent adenoviruses (RCAs) when E1 DNA is acquired by homologous recombination between overlapping sequences con-tained in the adenoviral DNA inserted in chromoso-mal DNA of the 293 cells and those present in the vector backbone. Producer cells, like PERC.6 and N52.6, contain minimal E1 sequences to avoid overlap with vector DNA to prevent RCA production (Lusky, 2005).
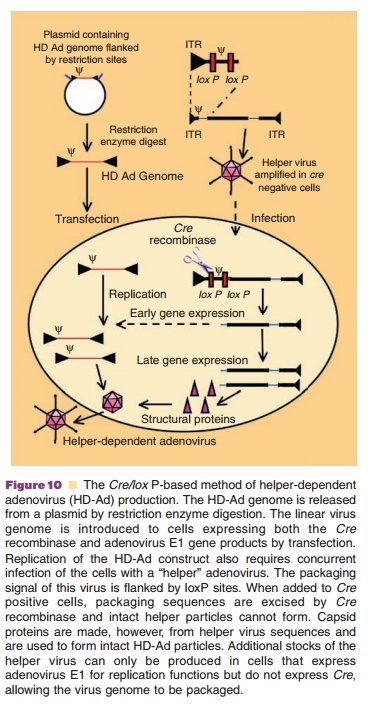
Production of helper-dependent virus that only contains viral ITRs and packaging signal requires a system that expresses the entire adenovirus genomeintrans. Due to the complexity of the viral genome, sucha system has not yet been constructed. The most effective production system for helper-dependent adenoviruses requires the use of a first generation E1-deleted adenovirus to supply all the necessary genes to support vector DNA amplification and packaging into viral capsids. Infection of 293 cells expressing the Cre recombinase (293Cre) with a helper virus with packaging elements framed by loxP sites leads to efficient removal of their packaging elements after Cre-mediated recombination at the loxP recogni-tion sequences (Fig. 10). This and cesium chloride (CsCl) density gradient ultracentrifugation has re-duced helper virus contamination levels to 0.1% to 0.01%. Use of the Cre/loxP or FLP/frt recombinase systems in cells other than 293 have further reduced RCA and helper contamination to 0.004% to 0.1% (Goncalves, 2006).
Recombinant adenoviral vectors were often purified by CsCl density gradient ultracentrifugation. This is still used for research-scale preparations, but is neither scalable nor efficient for large quantities of clinical grade virus. The most recent scalable purifica-tion methods use anion exchange chromatography due to the strong affinity of intact virus particles for the resin with respect to that of cellular and individual capsid proteins. Published loading estimates for anion exchange resins range from 0.5 to 5x1012 virus particles/mL of resin or 0.14 to 1.4 mg of virus/mL resin (Burova, 2005). Gel filtration and immobilized metal affinity chromatography are often used in polishing steps following anion exchange purification of recombinant adenovirus-based products.
The most significant effort to develop formula-tions to stabilize viruses at ambient temperatures for gene transfer has focused on the adenovirus. Until recently, it was assumed that lyophilization of adenovirus preparations would be the only way toachieve long-term stability. During the period between 1979 and 1988, numerous reports described liquid formulations that could confer long-term stability of the virus at –20˚C and –80˚C but very few described stability at 4˚C or room temperature (Altaras, 2005). Within the last 6 years, however, an extensive amount of information has been generated in the context of adenovirus stability. Evans et al. and others found that surface adsorption, freeze-thaw damage and free-radical oxidation were the primary inactivation pathways for recombinant adenoviruses (Croyle, 1998; Quick, 2002; Evans, 2004). Use of non-ionic surfactants such as polysorbate-80 to prevent adsorption to glass surfaces, cryoprotectants such as sucrose to prevent damage associated with changes in solution pH and virus confirmation during freeze-thaw cycles and free-radical oxidation inhibitors such as histidine, ethanol and ethylenediaminetetraacetic acid (EDTA) can confer a shelf life of 2 years for certain virus preparations at 4˚C. The role of other formulation variables, such as virus concentration, purity, divalent cations and solution pH on adeno-virus stability profiles has also been described (Altaras, 2005; Rexroad, 2006).
One of the most significant challenges for adenovirus-based products is the lack of sophisti-cated techniques for final characterization. Some progress has been made in this area with the recent finding that the penton base and protein IIIa are significantly more labile than other virus capsid proteins (Rexroad, 2003), suggesting that structural transitions in these regions should be monitored during the screening and selection of candidate formulations for long-term stability. It was also originally thought that the virus was much too complex for stability data to follow an Arrhenius relationship. However, using highly sensitive bio-logical and physicochemical methods, it is now clear that adenovirus degradation is a first-order process (Evans, 2004). This provides a platform for rational selection of formulation candidates and suggests that data from short-term accelerated stability studies is useful for predicting the rate of degradation during long-term storage. It is also clear that formulations for recombinant adeno-viruses will most likely have to be vector and process specific and tailored to specific nuances associated with a process that makes the virus susceptible to shearing stressors, aggregation and surface adsorption. They will also need to be serotype specific based on the fact that there are significant differences in the amino acid sequences of capsid proteins between the adenovirus groups. These tasks will most likely require significant collaborative efforts between the process engineer and the pharmaceutical scientist.
Related Topics