Chapter: 11th Biochemistry : Chapter 1 : Basic Concepts of Bio Chemistry and Cell Biology
Acid-Base Balance
Acid-Base Balance
The
normal pH of biological fluid is maintained in a narrow range. For example, the
pH of the blood is maintained between 7.35-7.40, i.e. slightly alkaline. The
changes in pH range will affect metabolic functions e.g. denaturation of
proteins, enzyme activity etc. Thus, maintenance of pH is vital for normal
physiological and biochemical functions of the body. The change of pH is due to
the change of acid-base concentrations in the cell and biological fluids.
Hence, the control and maintenance of acid-base balance is essential for the
maintenance of pH.
Regulation of Acid-Base balance
The
acid-base balance in the body is maintained by buffer system along with the
functions of lungs and kidney.
Role of Lung
The
first line of defense maintenance of pH is the control of extracellular
concentrations of CO2 and bicarbonate ions by the lungs. An increase
in ventilation removes CO2 from extracellular fluid which, in turn
reduces hydrogen ion concentration. Conversely, decreased ventilation increases
CO2, thus increasing hydrogen ion concentration in the extracellular
fluid. Both bicarbonate buffer system and hemoglobin buffer system of
erythrocytes are important for the maintenance of pH by lungs.
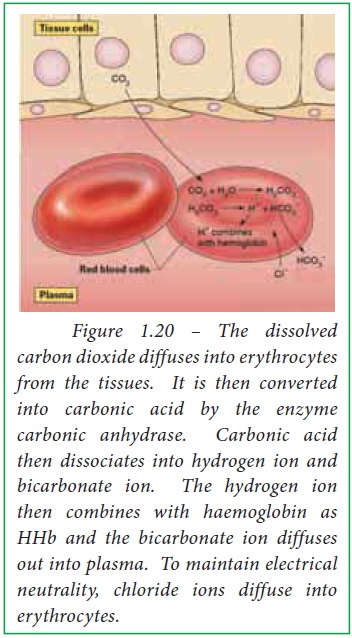
When blood passes through capillaries
of systemic circulation, carbonic acid is formed from diffused carbon dioxide
and water by the action of the enzyme carbonic anhydrase within the
erythrocytes. High carbonic acid concentration favors the dissociation of
carbonic acid into bicarbonate and hydrogen ions within erythrocytes. The
released H+ ions bind with deoxy haemoglobin whereas bicarbonate
ions diffuse out of erythrocytes into plasma. Thus haemoglobin reduces H+
ions within erythrocytes in tissues.
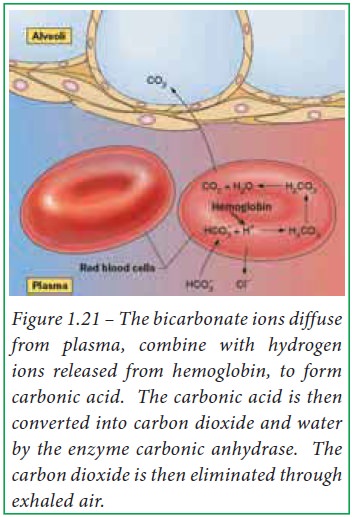
When blood reaches pulmonary
capillaries, deoxyhemoglobin is converted into oxyhemoglobin. The hydrogen ions
are released from hemoglobin because oxyhemoglobin has a weaker affinity for H+
ions. Due to this, bicarbonate ions diffuse into erythrocytes which, in turn
combine with H+ ions and form carbonic acid. At low partial pressure
of carbon dioxide, carbonic anhydrase converts carbonic acid into carbon
dioxide and water within erythrocytes. Thus, bicarbonate ions reduce H+
ions in lungs.
Role of Kidney
The kidney maintains pH by excreting
either acidic or basic urine. Excretion of acidic urine increases pH whereas
basic urine excretion decreases pH in extracellular fluid. Large amount of H+
ions are secreted into the tubular lumen by tubular epithelial cells. If they
are removed in urine, they will increase the pH of extracellular fluid. Large
amount of bicarbonate ions are also continuously secreted in the renal tubules.
If they are excreted in urine, then they reduce pH by retaining H+
ions. If more hydrogen ions are removed than bicarbonate ions, then there will
be a net loss of acid, whereas, if more bicarbonate ions are filtered than
hydrogen ions are secreted, then there will be a net loss of base. These
functions are achieved by mainly three components, namely, bicarbonate buffer
system, phosphate buffer system and ammonia. The role of these components are
as follows:
Bicarbonate buffer system in kidney
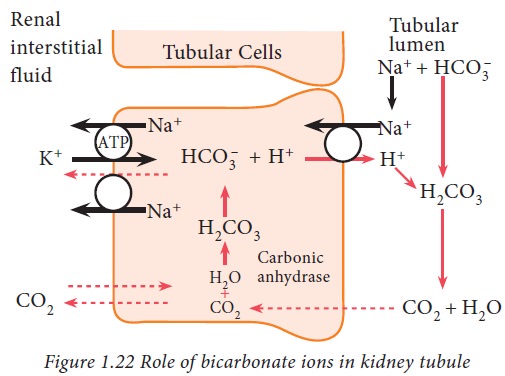
The
bicarbonate ions, freely filtered through glomerulus, combine with hydrogen
ions and form carbonic acid which, in turn, dissociates into carbon dioxide and
water. The carbon dioxide then diffuses into tubular cells where it again
combines with water to form carbonic acid in the presence of carbonic
anhydrase. Thus, bicarbonate and hydrogen ions are reabsorbed and retained.
This pattern of H+ ion secretion occurs in proximal convoluted
tubule, ascending loop of Henle and early part of distal convoluted tubule.
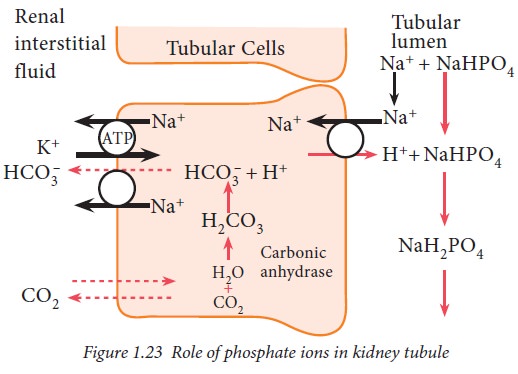
Phosphate buffer system in kidney
After the absorption of available
bicarbonate ions in tubular filtrate, remaining hydrogen ions interact with HPO24-
or NaHPO –4 and form H2PO4–
or NaH2PO4 which can be excreted in urine. Thus, hydrogen
ions are removed from extracellular fluid.
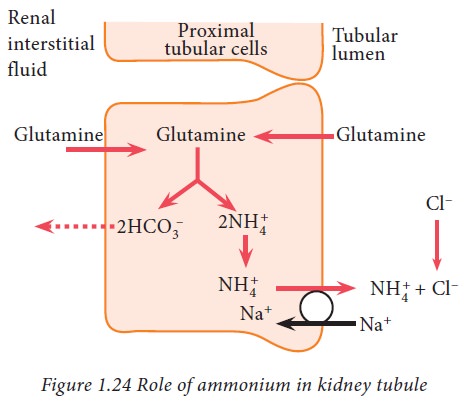
Ammonium buffer system in kidney
Ammonia produced from glutamine in
tubular epithelial cells, can freely diffuse into tubular lumen. This ammonia
then combines with hydrogen ions to form ammonium ions which can be easily
excreted in urine. Thus, hydrogen ions are removed from extracellular fluid.
Key Concept
Increased level of carbonic acid or
decreased level of bicarbonate ion results in acidosis. Decreased level of
carbonic acid or increased level of bicarbonate ion results in alkalosis.


Related Topics