Chapter: 11th Biochemistry : Chapter 1 : Basic Concepts of Bio Chemistry and Cell Biology
pH and Buffer system in Body fluids
pH and Buffer system in Body
fluids
Body fluid
All
parts of the body require nutrients and the metabolic wastes produced in them
need to be removed from the body. Hence, there is a need to transport various
substances like digested food materials, hormones, catabolites, enzymes,
various gases from one part of the body to another. These movements are
achieved through body fluids. In addition to this, body fluids also provide the
medium for the occurrence of metabolic reactions. Water is major component of
body fluids. Water is present within and around the cells of the body, and
within all the blood vessels. The total body water (TBW) is approximately 60%
of body weight.

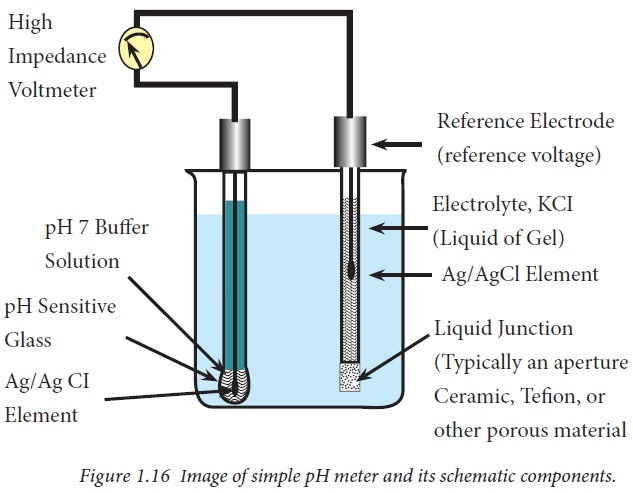
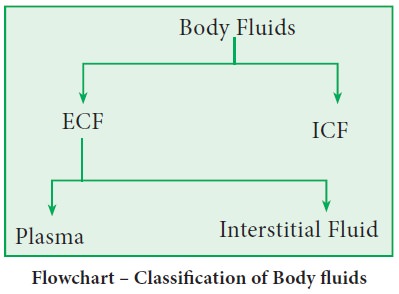
Body
fluids are watery solution of dissolved substances such as oxygen, nutrients
and wastes etc. Depending upon their location (compartment), they are of two
types, namely, intracellular fluid (ICF) and extracellular fluid (ECF).
Intracellular fluid is the fluid present within all the cells of the body.
Intracellular fluid is two thirds of TBW i.e. 40% of body weight. The major
cations of ICF are K+ and Mg2+. The major anions are proteins and organic
phosphates.
Collectively,
the fluid present in the blood and in the spaces surrounding cells is called
extracellular fluid (ECF), that is, all the fluid that is outside of cells. The
ECF is one thirds of TBW i.e. 20% of body weight. The major cation is Na+. The
major anions are Cl– and HCO3–. The ECF is comprised of plasma (1/4th of ECF) and
interstitial fluid (3/4th of ECF).
The
interstitial fluid (tissue fluid) lies around and between cells. Its
composition is the same as that of plasma except that it lacks larger proteins.
Thus, interstitial fluid is an ultrafiltrate of plasma. Cerebrospinal fluid and
lymph are examples for interstitial fluid.
Cerebrospinal fluid (CSF)
The
cavities of the brain (ventricles), the spinal cord and subarachnoid region is
filled with CSF. The total volume of CSF is 100 – 150 ml. It is a clear,
transparent and colorless fluid. It has similar pH as that of blood (7.20 to
7.40 i.e. slightly alkaline). It protects the brain and spinal cord from shocks
and maintains a uniform pressure on the nervous structures. It acts as a
reservoir to regulate the contents of the cranium. To a limited extent, it acts
as a medium for nutrient exchange in the nervous system.
Lymph
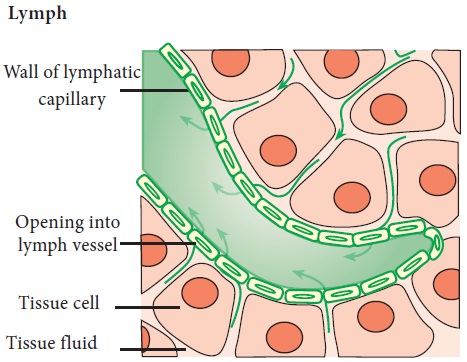
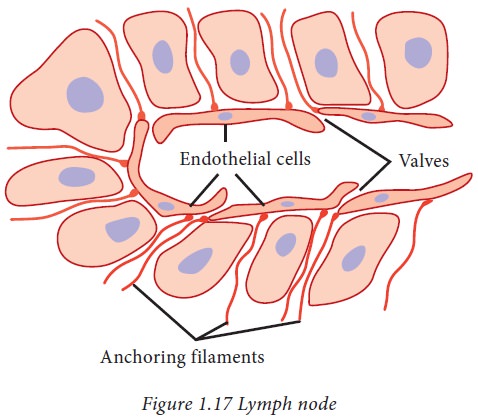
Formation and flow of lymph and interstitial fluid
The
fluid present in the lymphatic system is called lymph. Lymph is a colorless
fluid. It is composed of fluid matrix, plasma and leucocytes. It bathes tissues
and organs in its protective covering. There are no erythrocytes in lymph and
it has lower protein content than blood. Its pH is as same as that of blood
(7.35 to 7.40 i.e. slightly alkaline).
When the
blood passes through the capillaries in tissues, some water, along with many
small water-soluble substances, move out into the spaces between the cells.
Larger proteins and most of the formed
elements are left in the blood vessels. This fluid is called the interstitial
fluid or tissue fluid. Exchange of nutrients, gases, etc. between the blood and
the cells always occur through this fluid. There is an elaborate network of
vessels called the lymphatic system. The lymphatic system collects this lymph
fluid and drains it back to the major veins such as thoracic duct and
subclavian vein.
Fats are absorbed through lymph in
the lacteals present in the intestinal villi. Lymph drains fluid from
extracellular or intercellular spaces into blood. It is used to maintain a
balance between blood and interstitial fluid.
Blood
Blood functions as a vehicle for mass
transport of materials between cells and the environment or between the cells
themselves for maintaining homeostasis. Blood consists of cellular portion
called as formed elements (cells) that are suspended and carried in a fluid
portion called plasma. The total blood volume in an adult is about 5 litres.
The normal pH range of the blood is 7.35 – 7.40.
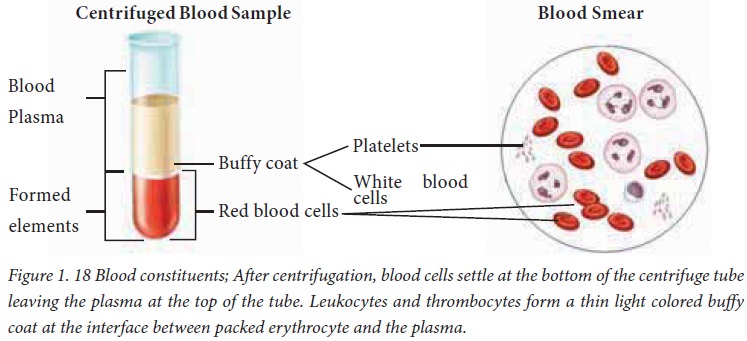
When a blood sample is centrifuged,
the heavier formed elements are packed at the bottom of the centrifuge tube,
leaving plasma at the top. The formed elements constitute 45% of total blood
volume and the plasma accounts for the remaining 55%.
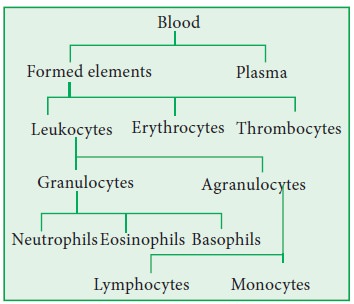
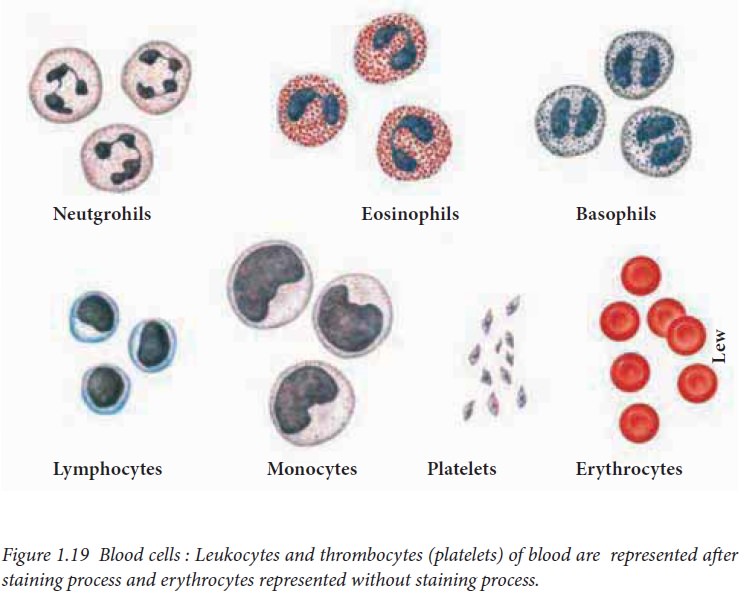
The formed elements include erythrocytes,
leukocytes and platelets (thrombocytes). A cubic millimeter of adult blood
normally contains 4.9 million to 5.5 million erythrocytes in males and 4.4
million to 5.0 million erythrocytes in females. The number of leukocytes in an
adult human being is 5000 to 9000 leukocytes per cubic millimeter.
Leukocytes include granulocytes
(neutrophils, eosinophils, basophils) and agranulocytes (lymphocytes and
monocytes). The normal platelet count in the blood is between 150000 and 300000
cells per cubic millimeter.
Plasma
is a pale-yellow coloured liquid consisting of water and dissolved solutes. The
solutes include ions like Na+ as well as organic molecules such as
metabolites, hormones, enzymes, albumins, globulins, fibrinogen and other
proteins.
Blood performs the following functions:
a. Blood transports oxygen from lungs to the tissues and carbon
dioxide from tissues to the lungs.
b. It transports absorbed nutrients from digestive tract to all the
body tissues.
c.
It transports
metabolic waste materials to kidney, lungs, skin and intestine for their
removal.
d. It transports various minerals, vitamins and hormones.
e.
It regulates water
balance.
f.
It maintains
acid-base balance in the body.
g. It provides defense against various infections through
leukocytes and antibodies.
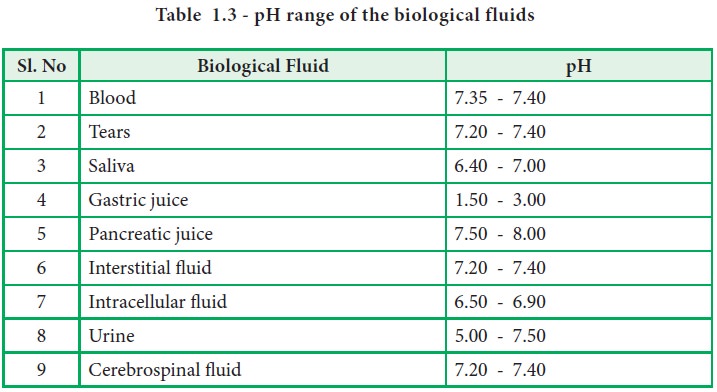
The pH
of important biological fluids is presented in the following table (Table 1.3).
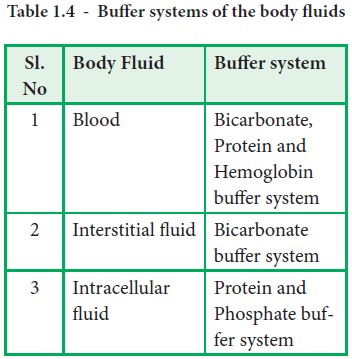
Various Buffers of Blood
Blood
contains four buffers namely
·
Bicarbonate buffer system
·
Phosphate buffer system
·
Protein buffer system
·
Hemoglobin buffer system
Bicarbonate buffer system
The bicarbonate buffer system
consists of carbonic acid and bicarbonate ions. The pKa of the
bicarbonate buffer system is 6.1. It is the most important buffer system of blood
plasma. The carbon dioxide released during fuel metabolism reacts with water by
the
action of the enzyme carbonic anhydrase to form carbonic acid [H2CO3].
Carbonic acid is a weak acid that partially dissociates into bicarbonate 1ion
[HCO3–] and H+ ion.
CO2 + H2O ↔ H2CO3 ↔
H+ + HCO–3
As base is added and H+
removed, carbonic acid dissociates into hydrogen ion and bicarbonate ions, and
dissolved CO2 reacts with water to replenish the carbonic acid
levels. When CO2 levels are increased, it forms more amount of carbonic
acid which in turn dissociates into hydrogen ion and bicarbonate ions. Thus
bicarbonate buffer functions as buffer system in blood.
Phosphate buffer system
The dihydrogen phosphate ions and
monohydrogen phosphate [HPO42–] ions contribute to
the phosphate buffer system. The pKa of a phosphate buffer system is
6.8. Phosphoric acid dissociates into H+ ions and dihydrogen
phosphate [H2PO4–] ions with pKa of
2.15. Dihydrogen phosphate [H2PO4–] ion
dissociates into H+ ions and monohydrogen phosphate [HPO42–]
ions with pKa of 7.2 whereas monohydrogen phosphate ions dissociates
into hydrogen ion and phosphate PO43– anions with pKa
of 12.4. From the dissociation constant values, it is clearly understood that
phosphate acts as an effective buffer in blood (pH = 7.4).
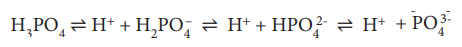
But,
phosphate concentration is very low in blood, thus, phosphate buffer, plays a
major role as an intracellular buffer in red blood cell and other types of
cells where their concentrations are higher than in blood and interstitial
fluid. The sodium salts of phosphoric acid also act as buffer system.
Protein buffer system
Plasma
proteins are responsible for protein buffer system. The buffering capacity of
proteins depends upon the pKa of ionisable group of aminoacid side
chains. Histidine residue plays a vital role as buffering agent because its
imidazole group pKa value is 6.7 and it is the more effective
contributor for protein buffer system. The plasma proteins are responsible for
the 2% buffering capacity of plasma. At blood pH 7.4, proteins exist as anions
(Pr–) serving as conjugate base. After accepting H+ ions
it is converted into weak acid (HPr). Thus, buffering action of proteins is due
to the following dissociation reaction:
HPr ↔ Pr–
+ H+
Protein-H ↔ Protein– + H+
Hemoglobin buffer systems
Hemoglobin
present in erythrocytes also plays an important role as buffering agent. It
mainly buffers the acids produced during gaseous transport between lungs and
tissues.
HHb ↔ Hb– + H+
At
tissue levels, H+ ions released from carbonic acid bind with
haemoglobin and help in the transport of CO2 as HCO3–.
In lungs, as haemoglobin combines with oxygen, it releases H+ ions,
which in turn bind with HCO3– to form carbonic acid.
Carbonic acid then dissociates into CO2 and water. Then, CO2
is exhaled. Thus haemoglobin acts as a buffer system.
Related Topics