Chapter: 11th Biochemistry : Chapter 1 : Basic Concepts of Bio Chemistry and Cell Biology
Hydrogen ion concentration and pH
Hydrogen ion concentration and pH
The acidic or basic nature of a solution is measured by H+ ion concentration. The conventional units such as moles/l or g/l are not commonly used to express H+ ion concentration. Sorenson (1909) introduced the term pH to express H+ ion concentration. pH is defined as the negative logarithm of H+ ion concentration.
pH = - log [H+]
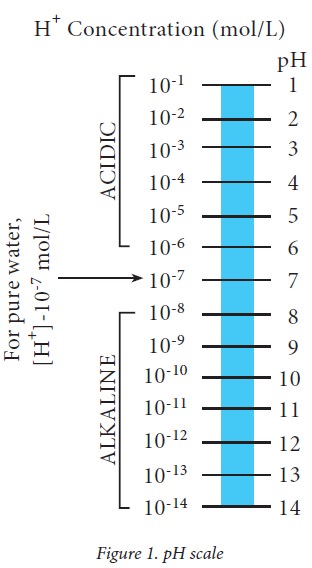
pH Scale
The pH is a narrow scale, ranging from 0 to 14 which corresponds to 1 M solution to 10-14 M solution of [H+] . Pure water has an equal concentration of H+ and OH– ions i.e. 10-7 M each. Thus, pure water has a pH 7 which is neutral. Solutions with pH less than 7 are said to be acidic while those with pH greater than 7 are alkaline. lt must be remembered that the term acidic or alkaline are not absolute but only relative. Thus, a solution with pH 3.0 is more acidic when compared with a solution of pH 4.5. A rise in H+concentration decreases pH while a fall in H+ concentration increases pH. The reverse is true for OH– concentration. The pH of a solution containing 1N [H+] is 0 while that containing 1N [OH–] is 14.
H+ Concentration (mol/L)

Related Topics