Water | Chapter 13 | 8th Science - Water - A Universal Solvent | 8th Science : Chapter 13 : Water
Chapter: 8th Science : Chapter 13 : Water
Water - A Universal Solvent
Water - A Universal
Solvent
A solvent is a substance which
dissolves other substances (solute). For example, in a salt solution, water is
the solvent and salt is the solute. Water has a unique property to dissolve
more substances than any other solvents. It can dissolve solids such as salt
and sugar, liquids such as honey and milk and gases such as oxygen and carbon
dioxide in it. Therefore, it is called as universal solvent.
Activity 3
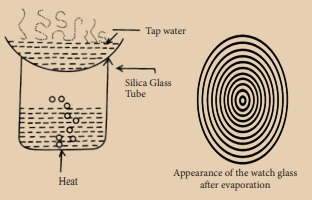
Place a sample of tap
water on a clean watch glass and place it over a beaker containing water, as
shown in the figure. Boil the water in the beaker. When all the water has
evaporated from the watch glass, remove it from the burner and let it cool.
What do you see on the watch glass?
Answer:
(i) We can observe a number of concentric rings of solid matter
deposited on the watch glass.
(ii) These are the dissolved solids left behind after the
evaporation of water. Salts, minerals and impurities are the solids dissolved
in water.
You can see a number of concentric
rings of solid matter deposited on the watch glass. These are the dissolved
solids left behind after the evaporation of water. Salts, minerals and
impurities are the solids dissolved in water. Dissolved salts are important for
the following reasons.
* They are essential for the growth
and development of plants.
* They add taste to water.
* They supply the essential minerals
needed for our bodies.
* Most of the chemical reactions
important for our living take place in the cells of our body with the help of
water.
Tap water, river water
and well water contain dissolved solid but rainwater and distilled water do not
contain dissolved solids. Hence concentric rings are not formed in the rain
water and distilled water after evaporation.
Apart from solids and minerals, air
is also dissolved in water. Air is present in dissolved state in all natural
sources of water. The solubility of oxygen in water is higher than the
solubility of nitrogen. Air dissolved in water contains approximately 35. 6%
oxygen along with nitrogen and carbon dioxide. Air being dissolved in water is
important for the following reasons.
* Air dissolved in water is
important for the living organisms to survive.
* Fish extracts the oxygen from the
water and expels water through the gills. Fish can survive in water only
through the dissolved oxygen present in water.
* Aquatic plants make use of dissolved carbon
dioxide for photosynthesis
* Carbon dioxide dissolved in water
reacts with limestone to form calcium bicarbonate. Marine organisms such as
snails, oysters, etc. , extract calcium carbonate from calcium bicarbonate to
build their shells.

Activity 4
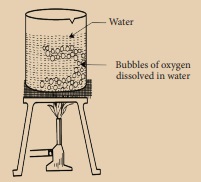
Take a beaker and fill
it half with fresh tap water and heat it. You will see small bubbles appearing
on the side of the beaker long before the water reaches its boiling point.
These bubbles are oxygen gas dissolved in water.
Related Topics