Water | Chapter 13 | 8th Science - Preparation of Water | 8th Science : Chapter 13 : Water
Chapter: 8th Science : Chapter 13 : Water
Preparation of Water
Preparation of Water
Water was first prepared in 1781 by
an English scientist Henry Cavendish. He discovered hydrogen gas when active
metals reacted with sulphuric acid. The hydrogen gas released was highly
inflammable and burnt to form a colourless product called water.
Zn + H2SO4 → ZnSO4
+ H2↑
2H2 + O2 → 2H2O
Water is also produced by the reduction of metal oxide by hydrogen, burning of hydrogen in air and burning of hydrocarbons in air. Respiration of plants and animals also releases water.
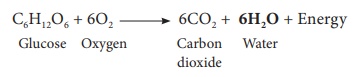
C6H12O6
+ 6O2 → 6CO2 + 6H2O + Energy
Glucose + Oxygen → Carbon dioxide + Water
+ Enegy
Henry Cavendish was a British philosopher,
scientist, chemist, and physicist. Cavendish is noted for his discovery of
hydrogen. He called it inflammable air. He mixed metals with strong acids and created
hydrogen. He created carbon dioxide also by combining metals with strong bases.

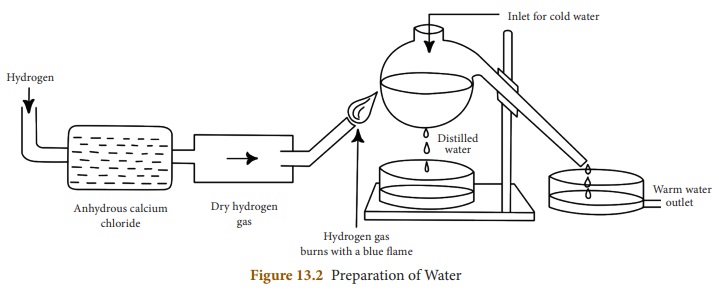
Laboratory preparation
of water
The apparatus used for the
preparation of water in the laboratories is as shown in Figure 13. 2. In this
method, pure hydrogen gas is passed through anhydrous calcium chloride to
absorb water vapour, if present. Dry hydrogen coming out of the opening is
burnt with sufficient supply of air. The burnt hydrogen gas forms droplets of
water, when it comes in contact with the cold flask. Distilled water without
any dissolved matter is obtained by this method.
Related Topics