Chapter: 8th Science : Chapter 13 : Water
Physical, Chemical of Properties of Water
Properties of Water
Water has some important properties which are familiar to us. But these properties are unique to water. Some of the physical and chemical properties are explained below.
Physical properties
a. Nature
Pure water is a clear and transparent liquid. It is colourless, odourless and tasteless.
b. Boiling point
The boiling point of water is 100°C at one atmospheric pressure (1 atm). At this temperature, water boils and changes into steam. The boiling point of water increases with increase in pressure. For example, when a pressure cooker is heated, a high pressure is built inside it. The high pressure increases the boiling point of water. Thus, water remains a liquid at a higher temperature (> 100°C) in the cooker. This cooks the food faster.
Pure water has the following physical properties.
• Pure water boils at 100° C at one atmospheric pressure.
• Pure water freezes at exactly 0°C at one atmospheric pressure.
• Pure water has a density of 1 gm/cm3
c. Freezing point
Water freezes at 0°C and forms ice. Thus, the freezing point of water is 0°C. The freezing point of water decreases with increase in pressure.
When the skaters move on ice,they exert pressure on it. This pressure lowers the freezing point. As a result, the ice melts underneaththe skate and allows the skaters to glide across the ice with little effort. When the skaters move forward, pressure is decreased and the water re-freezes to ice again.

d. Density
When ice cubes are put in a glass of water at room temperature, they float on the surface of the water. This is because ice is lighter than water. It means that the density of ice is lower than that of water. When the winter temperature is below 0°C, the water in the lake will start freezing. The frozen ice will float at the top and cover the lake. Since ice is a bad conductor of heat it does not allow heat to pass through it. So, the water below the ice remains in liquid form, where most of the aquatic life lives. This enables the aquatic animals and plants to survive even in extreme cold conditions. Density of water at different temperature is given in Table 13. 1.
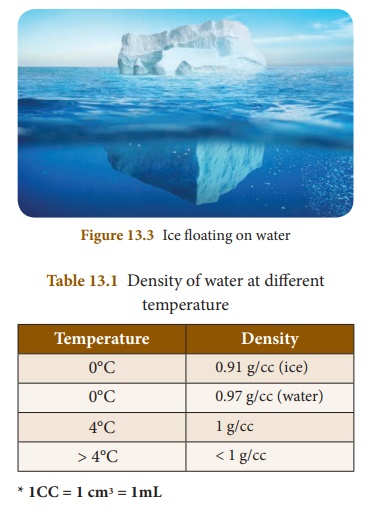
e. Anomalous expansion of water
For the same mass of ice and of water, the volume of ice is more than that of water. It is an unusual physical property of water. In the Himalayas the temperature can go down even below 0°C. The water in the water pipes will freeze at this temperature to ice. If the pipes are not strong they can crack, develop leaks or even burst. This is because freezing of water will cause an expansion in the volume.
f. Latent heat of fusion of ice
Take some ice cubes in a beaker and place a therometer in it. Now heat the beaker. The therometer will not register any rise in temperature till all the ice melts. The question arises where does the heat energy go if there is no rise in temperature. The heat energy is utilised in changing the state of ice from solid to liquid. The amount of heat energy required by ice to change into water is called latent heat of fusion of ice. Ice has the highest latent heat of fusion, i.e., 80 calories/g. or 336 J/g.
The freshness of fish and meat can be maintained by placing them in contact with ice. With its larger latent heat, ice is able to absorba large quantity of heat from the fish as it melts. Thus, food can be kept at a low temperature for an extended period of time.

g. Latent heat of vaporization of water
When water attains the temperature of 100°C, it starts changing its state from liquid to gaseous state. However, the temperature of water does not rise above 100°C. It is because the supplied heat energy only changes the state of the boiling water. This heat energy is stored in steam and is commonly called latent heat of vaporization of steam. The steam has the highest latent heat of vaporization and its value is 540 calories/g or 2268 J/g.
h. Specific heat capacity
The amount of heat that is needed to raise the temperature of a unit mass of a substance by 1°C is called specific heat capacity of that substance. The specific heat capacity of water is very high. One gram of water requires 1 calorie of heat to raise its temperature by 1°C. Due to its high specific heat capacity, water takes time to become hot as well as to cool down. Thus, water can absorb a lot of heat and retain it for a longer time. This property of water is used to cool engines. Water is circulated around car engine using the radiator pump and the heat is absorbed. Thus the engine is protected from getting too hot.

Chemical properties
a. Action towards litmus paper
Pure water is neutral and it shows no action towards litmus paper.
b. Stability
Water is a very stable compound. It does not decompose into elements, when heated to ordinary temperatures. However, if it is heated to 200°C, 0.02% of water decomposes to form hydrogen and oxygen gas.
2H2O --2000° C→ 2H2 + O2
c. Catalytic nature
Water acts as a catalyst in a number of reactions. Perfectly dry hydrogen and chlorine gases do not react in the presence of sunlight. However in the presence of traces of water, the reaction takes place with explosion to produce hydrogen chloride.

H2 + Cl2 Moisture Sunlight--→ 2HCl
d. Reaction with metals
Water reacts with some metals. Metals such as sodium, potassium and calcium react vigorously with water at room temperature. Sodium reacts with water to form hydrogen gas and sodium hydroxide solution. Due to the heat evolved in this reaction the hydrogen gas catches fire and burns.
2Na + 2H2O → 2NaOH + H2
Activity 2
Fill a trough with water. Cut a small piece of sodium with a knife and carefully drop it in the water. Sodium reacts with water and darts across the surface of water. A flame produced is also seen near the surface.
Magnesium is little more sluggish. It reacts with hot water and gives hydrogen and magnesium hydroxide solution.
Mg + 2H2O → Mg(OH)2 + H2↑
Many other metals react with water to form oxides and hydroxides. Iron is one such metal which forms iron oxide, called rust. Iron is used in many buildings, factories, bridges, ships and vehicles. The slow and gradual rusting of iron is called corrosion.
Copper does not react with water at any temperature. That is why it is used for making pipes and boilers.
e. Reaction with non-metals
Red hot carbon (coke) reacts with steam to produce water gas (Carbon monoxide + H2).
C + H2O --1000°C→ CO↑ + H2↑
Chlorine gas dissolves in water and produces hydrochloric acid.
2Cl2 + 2H2O --Sunlight→ 4HCl + O2
Related Topics