Water | Chapter 13 | 8th Science - Student Activities | 8th Science : Chapter 13 : Water
Chapter: 8th Science : Chapter 13 : Water
Student Activities
Activity 1
Take some anhydrous copper (II) sulphate powder and place it in a watch glass. Add water drop by drop to the anhydrous copper sulphate. Do you notice any colour change in the powder? You can notice the powder turning blue. It is a test for water.

Answer:
(i) The reaction between anhydrous copper (II) sulphate and water is used as a test for water.
(ii) The white solid turns blue in the presence of water.
Activity 2
Fill a trough with water. Cut a small piece of sodium with a knife and carefully drop it in the water. Sodium reacts with water and darts across the surface of water. A flame produced is also seen near the surface.
Activity 3
Place a sample of tap water on a clean watch glass and place it over a beaker containing water, as shown in the figure. Boil the water in the beaker. When all the water has evaporated from the watch glass, remove it from the burner and let it cool. What do you see on the watch glass?
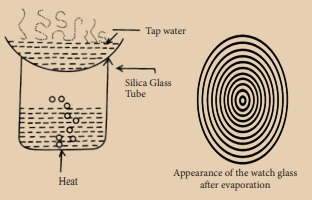
Answer:
(i) We can observe a number of concentric rings of solid matter deposited on the watch glass.
(ii) These are the dissolved solids left behind after the evaporation of water. Salts, minerals and impurities are the solids dissolved in water.
Activity 4
Take a beaker and fill it half with fresh tap water and heat it. You will see small bubbles appearing on the side of the beaker long before the water reaches its boiling point. These bubbles are oxygen gas dissolved in water.
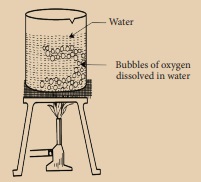
Activity 5
Take two pots with similar plants. Water one of the plants with tap water and the other with sea water. Record your findings and note the difference observed.
Answer:
Observation :
(i) Due to the high salinity of sea water, the plant starts to droop.
(ii) The plant which is watered with tap water grows well.
Activity 6
Take samples of water from different sources (like a tube well, a lake, a pond or a river) and pour equal quantities of each sample of water into different test tubes. Measure the height of water in each test tube with a scale. Add one or two drops of liquid soap to each test tube.
Shake each test tube five times and observe the height of the lather in each sample. Record your observations in the table. Which water is soft? Which water is hard? Can you say why?
Answer:
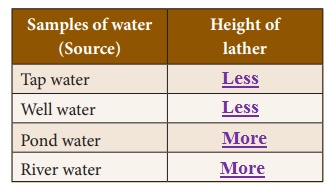
Samples of water (Source) : Height of lather
1. Tap water : Less
2. Well water : Less
3. Pond water : More
4. River water : More
Which water is soft? Can you say why?
Answer:
(i) Pond and river water are considered as soft due to presence of very small quantities of salts.
(ii) Salts are present in very small quantities .
Which water is hard? Can you say why?
Answer: Tap and well water are considered as hard due to presence of large quantity of minerals and salts .
Activity 7
Take a shampoo, shower gel or other product you think might have micro-beads in it. Mix two tablespoons of this in a glass of water and stir it well. Pour the water in a black t-shirt filtering the micro-beads out.

Henry Cavendish was a British philosopher, scientist, chemist, and physicist. Cavendish is noted for his discovery of hydrogen. He called it inflammable air. He mixed metals with strong acids and created hydrogen. He created carbon dioxide also by combining metals with strong bases.

Pure water has the following physical properties.
• Pure water boils at 100° C at one atmospheric pressure.
• Pure water freezes at exactly 0°C at one atmospheric pressure.
• Pure water has a density of 1 gm/cm3
When the skaters move on ice,they exert pressure on it. This pressure lowers the freezing point. As a result, the ice melts underneaththe skate and allows the skaters to glide across the ice with little effort. When the skaters move forward, pressure is decreased and the water re-freezes to ice again.

The freshness of fish and meat can be maintained by placing them in contact with ice. With its larger latent heat, ice is able to absorba large quantity of heat from the fish as it melts. Thus, food can be kept at a low temperature for an extended period of time.

Copper does not react with water at any temperature. That is why it is used for making pipes and boilers.
Tap water, river water and well water contain dissolved solid but rainwater and distilled water do not contain dissolved solids. Hence concentric rings are not formed in the rain water and distilled water after evaporation.
The salinity of water is more in the Dead sea. It is actually a salt lake as it has a single source of water and is not connected to the ocean. It is landlocked and this causes the water to evaporate. This has led to a steady increase in its degree of salinity. Now the salinity is so high such that the marine life cannot survive in it. This is why it is called the Dead sea.

Every year 4. 6 million childrendie due to diarrhea. Access to clean water improves hygiene and health.
RO purifiers are the purifiers that can remove the dissolved impurities and germs. They also improve the taste of water. RO standsfor the name of the technology, ‘reverse osmosis’, used in these purifiers. Some RO purifiers also have a UV (ultraviolet) unit that destroys the germs present in water.
Distilled water and boiled water have no taste. The pleasant taste of drinking water is due to the presence of dissolved substances which include air, carbon dioxide and minerals.
About 90% of the available surface water has already been tapped mainly for agriculture and irrigation.
The largest source of wate pollution in India is untreated sewage. On an average, a person uses 135 litres of water per day for washing clothes, cooking, bathing, etc.
Plastic sheets are used in agriculture to grow vegetables. At the end of the season, these plastic sheets are ploughed back into thesoil. The plastic sheets break into tiny pieces and get eaten by earth worms, which is harmful to their health and that of soil.
Micro-plastics can be found in almost every freshwater source. They have been found from the freezing waters of the Arctic andAntarctic to the bottom of the deep-sea floor upto 5,000 meters deep. Micro-plastics have been found in bottled water and tap water around the world.

Related Topics