Chapter: Medical Physiology: The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Volume and Osmolality of Extracellular and Intracellular Fluids in Abnormal States
Volume and Osmolality of Extracellular and Intracellular Fluids in Abnormal States
Some of the different factors that can cause extracellular and intracellular volumes to change markedly are ingestion of water, dehydration, intravenous infusion of different types of solutions, loss of large amounts of fluid from the gastrointestinal tract, and loss of abnor-mal amounts of fluid by sweating or through the kidneys.
One can calculate both the changes in intracellular and extracellular fluid volumes and the types of therapy that should be instituted if the following basic principles are kept in mind:
Water moves rapidly across cell membranes; therefore, the osmolarities of intracellular and extracellular fluids remain almost exactly equal to each other except for a few minutes after a change in one of the compartments.
Cell membranes are almost completely impermeable to many solutes; therefore, thenumber of osmoles in the extracellular or intracellular fluid generally remains constant unless solutes are added to or lost from the extracellular compartment.
With these basic principles in mind, we can analyze the effects of different abnormal fluid conditions on extracellular and intracellular fluid volumes and osmolarities.
Effect of Adding Saline Solution to the Extracellular Fluid
If an isotonic saline solution is added to the extracel-lular fluid compartment, the osmolarity of the extra-cellular fluid does not change; therefore, no osmosis occurs through the cell membranes. The only effect is an increase in extracellular fluid volume (Figure 25–6A). The sodium and chloride largely remain in the extracellular fluid because the cell membrane behaves as though it were virtually impermeable to the sodium chloride.
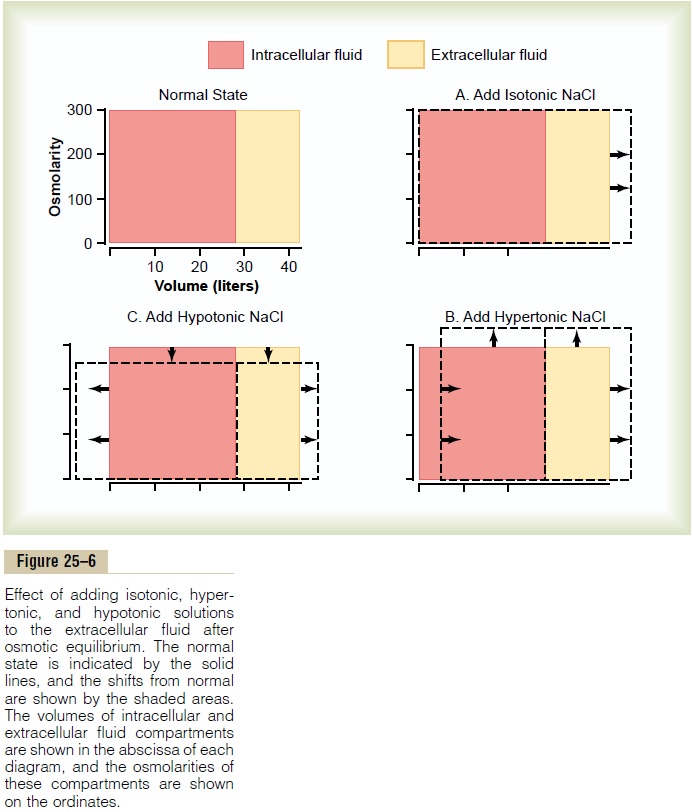
If a hypertonic solution is added to the extracellular fluid, the extracellular osmolarity increases and causes osmosis of water out of the cells into the extracellular compartment (see Figure 25–6B). Again, almost all the added sodium chloride remains in the extracellular compartment, and fluid diffuses from the cells into the extracellular space to achieve osmotic equilibrium. The net effect is an increase in extracellular volume (greater than the volume of fluid added), a decrease in intracellular volume, and a rise in osmolarity in both compartments.
If a hypotonic solution is added to the extracellular fluid, the osmolarity of the extracellular fluid decreases and some of the extracellular water diffuses into the cells until the intracellular and extracellular compart-ments have the same osmolarity (see Figure 25–6C). Both the intracellular and the extracellular volumes are increased by the addition of hypotonic fluid, although the intracellular volume increases to a greater extent.
Calculation of Fluid Shifts and Osmolarities After Infusion of Hypertonic Saline. We can calculate the sequentialeffects of infusing different solutions on extracellular and intracellular fluid volumes and osmolarities. For example, if 2 liters of a hypertonic 3.0 per cent sodium chloride solution are infused into the extracellular fluid compartment of a 70-kilogram patient whose initial plasma osmolarity is 280 mOsm/L, what would be the intracellular and extracellular fluid volumes and osmolarities after osmotic equilibrium?
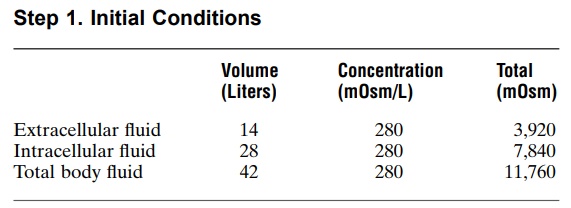
The first step is to calculate the initial conditions, including the volume, concentration, and total milliosmoles in each compartment. Assuming that extracellular fluid volume is 20 per cent of body weight and intracellular fluid volume is 40 per cent of body weight, the following volumes and concentrations can be calculated.
Next, we calculate the total milliosmoles added to the extracellular fluid in 2 liters of 3.0 per cent sodium chloride. A 3.0 per cent solution means that there are 3.0 g/100 ml, or 30 grams of sodium chloride per liter. Because the molecular weight of sodium chloride is about 58.5 g/mol, this means that there is about 0.513 mole of sodium chloride per liter of solution. For 2 liters of solution, this would be 1.026 mole of sodium chloride. Because 1 mole of sodium chloride is about equal to 2 osmoles (sodium chloride has two osmoti-cally active particles per mole), the net effect of adding 2 liters of this solution is to add 2051 milliosmoles of sodium chloride to the extracellular fluid.
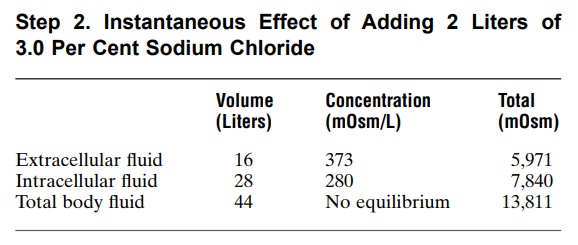
In Step 2, we calculate the instantaneous effect of adding 2051 milliosmoles of sodium chloride to the extracellular fluid along with 2 liters of volume. There would be no change in the intracellular fluidconcen-tration or volume, and there would be no osmotic equilibrium. In the extracellular fluid, however, there would be an additional 2051 milliosmoles of total solute, yielding a total of 5791 milliosmoles. Because the extracellular compartment now has 16 liters of volume, the concentration can be calculated by divid-ing 5791 milliosmoles by 16 liters to yield a con-centration of 373 mOsm/L. Thus, the following values would occur instantly after adding the solution.
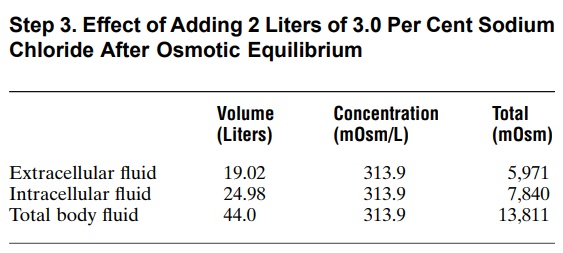
In the third step, we calculate the volumes and con-centrations that would occur within a few minutes after osmotic equilibrium develops. In this case, the concentrations in the intracellular and extracellular fluid compartments would be equal and can be calcu-lated by dividing the total milliosmoles in the body, 13,811, by the total volume, which is now 44 liters. This yields a concentration of 313.9 mOsm/L. Therefore, all the body fluid compartments will have this same con-centration after osmotic equilibrium. Assuming that no solute or water has been lost from the body and that there is no movement of sodium chloride into or out of the cells, we then calculate the volumes of the intracellular and extracellular compartments. The intracellular fluid volume is calculated by dividing the total milliosmoles in the intracellular fluid (7840) by the concentration (313.9 mOsm/L), to yield a volume of 24.98 liters. Extracellular fluid volume is calculated by dividing the total milliosmoles in extracellular fluid (5971) by the concentration (313.9 mOsm/L), to yield a volume of 19.02 liters. Again, these calculations are based on the assumption that the sodium chloride added to the extracellular fluid remains there and does not move into the cells.
Thus, one can see from this example that adding 2 liters of a hypertonic sodium chloride solution causes more than a 5-liter increase in extracellular fluid volume while decreasing intracellular fluid volume by almost 3 liters.
This method of calculating changes in intracellular and extracellular fluid volumes and osmolarities can be applied to virtually any clinical problem of fluid volume regulation. The reader should be familiar with such calculations because an understanding of the mathematical aspects of osmotic equilibrium between intracellular and extracellular fluid compartments is essential for understanding almost all fluid abnormal-ities of the body and their treatment.
Related Topics