Chapter: Medical Physiology: The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Osmotic Equilibrium Is Maintained Between Intracellular and Extracellular Fluids
Osmotic Equilibrium Is Maintained Between Intracellular and Extracellular Fluids
Large osmotic pressures can develop across the cell membrane with relatively small changes in the con-centrations of solutes in the extracellular fluid. As dis-cussed earlier, for each milliosmole concentration gradient of an impermeant solute (one that will not permeate the cell membrane), about 19.3 mm Hg osmotic pressure is exerted across the cell membrane.
If the cell membrane is exposed to pure water and the osmolarity of intracellular fluid is 282 mOsm/L, the potential osmotic pressure that can develop across the cell membrane is more than 5400 mm Hg. This demon-strates the large force that can move water across the cell membrane when the intracellular and extracellu-lar fluids are not in osmotic equilibrium. As a result of these forces, relatively small changes in the concen-tration of impermeant solutes in the extracellular fluid can cause large changes in cell volume.
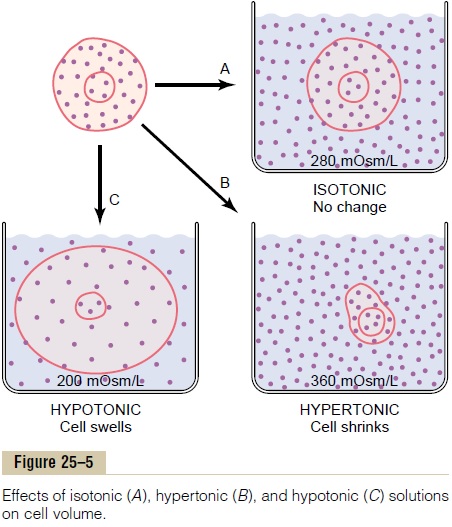
Isotonic, Hypotonic, and Hypertonic Fluids. The effects ofdifferent concentrations of impermeant solutes in the extracellular fluid on cell volume are shown in Figure 25–5. If a cell is placed in a solution of impermeant solutes having an osmolarity of 282 mOsm/L, the cells will not shrink or swell because the water concentra-tion in the intracellular and extracellular fluids is equal and the solutes cannot enter or leave the cell. Such a solution is said to be isotonic because it neither shrinks nor swells the cells. Examples of isotonic solutions include a 0.9 per cent solution of sodium chloride or a 5 per cent glucose solution. These solutions are impor-tant in clinical medicine because they can be infused into the blood without the danger of upsetting osmotic equilibrium between the intracellular and extracellu-lar fluids.
If a cell is placed into a hypotonic solution that has a lower concentration of impermeant solutes (less than 282 mOsm/L), water will diffuse into the cell, causing it to swell; water will continue to diffuse into the cell, diluting the intracellular fluid while also concentrating the extracellular fluid until both solutions have about the same osmolarity. Solutions of sodium chloride with a concentration of less than 0.9 per cent are hypotonic and cause cells to swell.
If a cell is placed in a hypertonic solution having a higher concentration of impermeant solutes, water will flow out of the cell into the extracellular fluid, con-centrating the intracellular fluid and diluting the extra-cellular fluid. In this case, the cell will shrink until the two concentrations become equal. Sodium chloride solutions of greater than 0.9 per cent are hypertonic.
Isosmotic, Hyperosmotic, and Hypo-osmotic Fluids. Theterms isotonic, hypotonic, and hypertonic refer to whether solutions will cause a change in cell volume. The tonicity of solutions depends on the concentration of impermeant solutes. Some solutes, however, can permeate the cell membrane. Solutions with an osmo-larity the same as the cell are called isosmotic, regard-less of whether the solute can penetrate the cell membrane.
The terms hyperosmotic and hypo-osmotic refer to solutions that have a higher or lower osmolarity, respectively, compared with the normal extracellular fluid, without regard for whether the solute permeates the cell membrane. Highly permeating substances, such as urea, can cause transient shifts in fluid volume between the intracellular and extracellular fluids, but given enough time, the concentrations of these sub-stances eventually become equal in the two compart-ments and have little effect on intracellular volume under steady-state conditions.
Osmotic Equilibrium Between Intracellular and Extracellular Fluids Is Rapidly Attained. The transfer offluid across thecell membrane occurs so rapidly that any differences in osmolarities between these two compartments are usually corrected within seconds or, at the most, minutes. This rapid movement of water across the cell membrane does not mean that complete equilibrium occurs between the intracellular and extracellular compartments throughout the whole body within the same short period. The reason for this is that fluid usually enters the body through the gut and must be transported by the blood to all tissues before complete osmotic equilibrium can occur. It usually takes about 30 minutes to achieve osmotic equilibrium everywhere in the body after drinking water.
Related Topics