Atomic Structure | Chapter 12 | 8th Science - Valency | 8th Science : Chapter 12 : Atomic Structure
Chapter: 8th Science : Chapter 12 : Atomic Structure
Valency
Valency
In order to understand valency of
elements clearly, we need to learn a little about Rutherford’s atomic model
here. According to Rutherford, an atom consists of subatomic particles namely,
proton, electron and neutrons. Protons and neutrons are found at the centre of
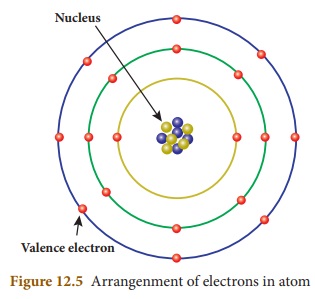
an atom, called nucleus. Electrons are revolving around the nucleus in a
circular path, called orbits or shells. An atom has a number of orbits and each
orbit has electrons. The electrons revolving in the outermost orbit are called
valence electrons.
The arrangement of electrons in the
orbits is known as electronic
configuration. Atoms of all the elements will tend to have a stable
electronic configuration, that is, they will tend to have either two electrons
(known as duplet) or eight electrons (known as octet) in their outermost orbit.
For example, helium has two
electrons in the outermost orbit and so it is chemically inert. Similarly, neon
is chemically inert because, it has eight electrons in the outermost orbit.
The valence electrons in an atom
readily participate in a chemical reaction and so the chemical properties of an
element are determined by these electrons. When molecules are formed, atoms
combine together in a fixed proportion because each atom has different
combining capacity. This combining capacity of an atom is called valency.
Valency is defined as the number of electrons lost, gained or shared by an atom
in a chemical combination so that it becomes chemically inert.
1. Types of Valency
As we saw earlier, an atom will
either gain or lose electrons in order to attain the stable electronic
configuration. In order to understand valency in a better way, it can be
explained in two ways depending on whether an atom gains or losses electrons.
Atoms of all metals will have 1 to 3
electrons in their outermost orbit. By loosing these electrons they will have
stable electronic configuration. So, they lose them to other atoms in a
chemical reaction and become positively charged. Such atoms which donate
electrons are said to have positive valency. For example, sodium atom (Atomic
number: 11) has one electron in its outermost orbit and in order to have
stability it loses one electron and becomes positively charged. Thus, sodium
has positive valency.
All non-metals will have 3 to 7
electrons in the outermost orbit of their atoms. In order to attain stable
electronic configuration, they need few electrons. They accept these electrons
from other atoms in a chemical reaction and become negatively charged. These
atoms which accept electrons are said to have negative valency. For example,
chlorine atom (Atomic number: 17) has seven electrons in its outermost orbit.
By gaining one electron it attains stable electronic configuration, like inert
gas electronic configuration. Thus, chlorine has negative valency.
2. Valency with respect
to atoms
Valency of an element is also
determined with respect to other atoms. Generally, valency of an atom is
determined with respect to hydrogen, oxygen and chlorine.
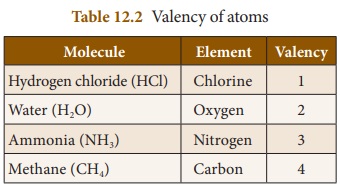
a. Valency with respect to Hydrogen
Since hydrogen atom loses one
elctron in its outermost orbit, its valency is taken as one and it is selected
as the standard. Valencies of the other elements are expressed in terms of
hydrogen. Thus, valency of an element can also be defined as the number of
hydrogen atoms which combine with one atom of it. In hydrogen chloride
molecule, one hydrogen atom combines with one chlorine atom. Thus, the valency
of chlorine is one. Similarly, in water molecule, two hydrogen atoms combine
with one oxygen atom. So, valency of oxygen is two.
Since some of the elements do not
combine with hydrogen, the valency of the element is also defined in terms of
other elements like chlorine or oxygen. This is because almost all the elements
combine with chlorine and oxygen.
b. Valency with respect to Chlorine
Since valency of chlorine is one,
the number of chlorine atoms with which one atom of an element can combine is
called its valency. In sodium chloride (NaCl) molecule, one chlorine atom
combines with one sodium atom. So, the valency of sodium is one. But, in
magnesium chloride (MgCl2) valency of magnesium is two because it
combines with two chlorine atoms.
c. Valency with respect to oxygen
In another way, valency can be
defined as double the number of oxygen atoms with which one atom of an element
can combine because valency of oxygen is two. For example, in magnesium oxide
(MgO) valency of magnesium is two.
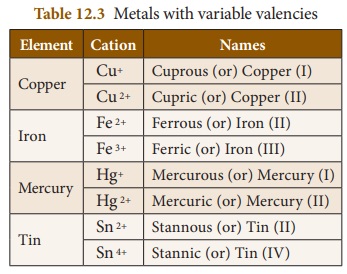
3. Variable Valency
Atoms of some elements combine with
atoms of other elements and form more than one product. Thus, they are said to
have different combining capacity. These atoms have more than one valency. Some
cations exhibit more than one valency. For example, copper combines with oxygen
and forms two products namely cuprous oxide (Cu2O) and cupric oxide
(CuO). In Cu2O, valency of copper is one and in CuO valency of
copper is two. For lower valency a suffix –ous is attached at the end of the
name of the metal. For higher valency a suffix –ic is attached at the end of
the name of the metal. Sometimes Roman numeral such as I, II, III, IV etc. indicated
in parenthesis followed by the name of the metal can also be used.
Related Topics