Atomic Structure | Chapter 12 | 8th Science - Points to Remember, Glossary, Concept Map | 8th Science : Chapter 12 : Atomic Structure
Chapter: 8th Science : Chapter 12 : Atomic Structure
Points to Remember, Glossary, Concept Map
Points to Remember
• An atom consists of elementary
particles like proton, electron and neutron.
• The discharge tube used in the
experiment is now referred as Crookes tube or Cathode Ray Tube (CRT). It is a long glass tube filled
with gas and sealed at both the ends.
• Different atoms have different
combining capacities. The combining capacity of an atom is known as its valency.
• Chemical formula is the short hand
notation of a molecule of a substance (compound) . It shows the actual number
of atoms of each element in a molecule of a substance.
• In naming a compound containing a
metal and a non-metal, the name of the metal is written first and the name of
the non-metal is obtained by adding the suffix-ide to its name.
• Balancing chemical equation is
necessary, so that law of conservation of mass may be obeyed.
• The law of conservation of mass
states that during any chemical change, the total mass of the products is equal
to the total mass of the reactants.
GLOSSARY
1.
Anode The positively
charged electrode or an electron acceptor.
2.
Cathode The negatively
charged electrode or an electron donor.
3.
Chemical formula It is a
representation of a substance using symbols for its constituent elements.
4.
Discharge tube A tube
containing charged electrodes and filled with a gas in which ionisation is
induced by an electric field.
5.
Ion An atom or molecule with a net
electric charge due to the loss or gain of one or more electrons.
6.
Molecular formula It is a
formula giving the number of atoms of each of the elements present in one
molecule of a specific compound.
7.
Precipitate An
insoluble solid that emerges from a liquid solution.
8.
Product A substance that is
formed as the result of a chemical reaction.
9.
Reactant A substance that
takes part in and undergoes change during a reaction.
10.
Valency The combining power
of an element, especially as measured by the number of hydrogen atoms it can
displace or combine with.
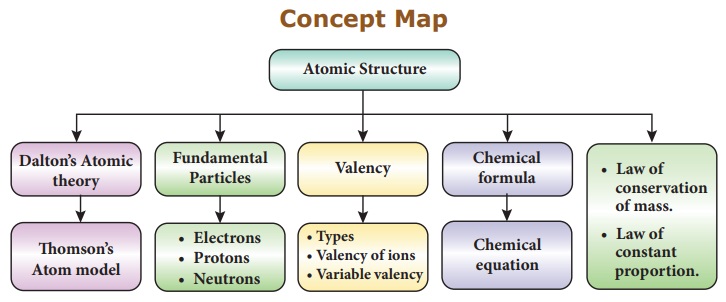
Concept Map
REFERENCE BOOKS
1. Petrucci, Ralph H et.al. General
Chemistry: Principles & Modern Applications (9th Edition). Upper Saddle
River, NJ: Pearson Prentice Hall, 2007. Print.
2. P.L.Soni, Text book of Inorganic
Chemistry,S. Chand publication, New Delhi
3. Complete Chemistry (IGCSE),
Oxford university press, New York
4. Raymond Chang. (2010).Chemistry.
New York, NY: The Tata McGraw Hill Companies. Inc.
5. Frank New Certificate Chemistry.
McMillan Publishers
INTERNET RESOURCES
1. https://www.chem4kids.com
2. https://courses.lumenlearning.com/
boundless - chemistr y/chapter/the - structure-of-the-atom/
3. https://www.khanacademy.org/science/ biology/chemistry--of-life/elements-and-atoms/e/atomic-structure
ICT
CORNER
ATOMIC
STRUCTURE
Through
this activity you will learn the atomic structure through Interactive games
Step 1
• Open the Browser and type the URL
given below.
• You can see Protons Neutrons and
Electrons Atom games.
• Click the first game, you will see
the periodic table. Start the quiz and answer it.
• Likewise explore the next game and
play it.
URL:
https://www.wartgames.com/themes/science/atomicstructure.html
Related Topics