Atomic Structure | Chapter 12 | 8th Science - Naming chemical compounds | 8th Science : Chapter 12 : Atomic Structure
Chapter: 8th Science : Chapter 12 : Atomic Structure
Naming chemical compounds
Naming chemical compounds
A chemical compound is a substance
formed out of more than one element joined together by chemical bond. Such
compounds have properties that are unique from that of the elements that formed
them. While naming these compounds specific ways are followed. They are given
below.
1. In naming a compound containing a
metal and a non-metal, the name of the metal is written first and the name of
the non-metal is written next after adding the suffix-ŌĆśideŌĆÖ to its name.
Examples:
NaCl - Sodium chloride
Ag Br - Silver bromide
2. In naming a compound containing a
metal, a non-metal and oxygen, name of the metal is written first and name of
the non-metal with oxygen is written next after adding the suffix- ŌĆśateŌĆÖ (for
more atoms of oxygen) or ŌĆō ite (for less atoms of oxygen) to its name.
Examples:
Na2 SO4 - Sodium
sulphate
Na NO2 - Sodium nitrite
3. In naming a compound containing
two non-metals only, the prefix mono, di, tri, tetra, penta etc. is written
before the name of non- metals.
Examples:
SO2 - Sulphur dioxide
N2O5 - Dinitrogen
pentoxide
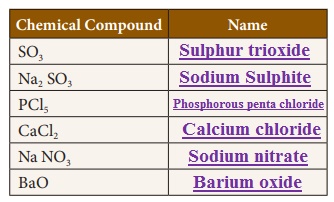
Activity 4
Write the names of the
chemical compounds.
Related Topics