Atomic Structure | Term 1 Unit 4 | 7th Science - Valency | 7th Science : Term 1 Unit 4 : Atomic Structure
Chapter: 7th Science : Term 1 Unit 4 : Atomic Structure
Valency
Valency
Imagine there are various people
having different pattern of hands. Some have no hands and some have one, some
two and others three. Few have four and no one has more than four.
The person with four hands can hold hands of four others at a same time, while
the one with no hands can never hold any hand. In this manner some atoms can
hold one electron,
some can hold two, some can hold three, some can hold four and some cannot hold
any electron. This property is called valency.
WHAT MAKES ATOMS STICK
TOGETHER?
Electrons carry a negative electric charge, and protons
carry a positive charge. The attraction between them holds electrons in orbits.
This combining property of an atom
is called as Valency. It is a measure of how many hydrogen atoms it can combine
with. For example: oxygen can combine with two hydrogen atoms and create water
molecule, the valency of oxygen atom is two. In case of chlorine, it can
combine with only one hydrogen to create HCl (hydrochloric acid) here the
valency of chlorine is one. Methane has one carbon atom combining with four
hydrogen atoms to form carbon molecule is methane (CH4). Can you
guess the valency of Carbon in methane? In ammonia molecule, Nitrogen combines
with three hydrogen atoms. What is the valency of Nitrogen in ammonia?
Valency is defined as the combining
capacity of an element. Atoms of different elements combine with each other to
form molecules. Valency determines the number of atoms of an element that
combines with atom or atoms of another type.
The element having valency one is
called monovalent. For example: Hydrogen and Sodium. The elements having
valency two are called divalent. For example: Oxygen and Beryllium. The
elements having valency three are called trivalent. For example: Nitrogen and
Aluminium. Some elements exhibits more than one valency. For example: Iron
combines with oxygen to form two types ferrous oxide (exhibits valency 2) and
ferric oxide (exhibits valency 3), however we will study about them later.
When atoms of different elements
combine with each other then molecules of compounds are formed. In these
instances, it is necessary to know the valancies of those elements. For
example:
1 2Na+ Cl2 ----------
2NaCl
Valency 1+ 1
Here, the valancies of both sodium
and chlorine are 1.
Remember The valency of element Na
is 1
The valency of element Cl is 1
Then, the molecular formula will be
Symbol of Elements Na Cl Molecular
Formula
Radicals and ions11
NaCl
2 Mg + Cl2---------- Mg
Cl2
Valency 2 1
Here , the valency of magnesium is 2
and that of Cl is 1.
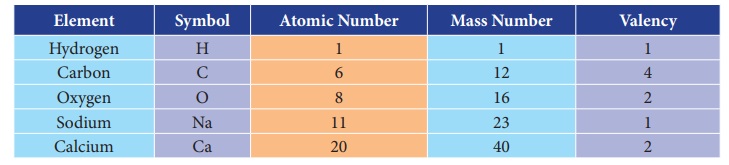
Elements and their symbols with
their atomic number and mass number and valency.
Related Topics