Atomic Structure | Term 1 Unit 4 | 7th Science - Atomic number and Mass number | 7th Science : Term 1 Unit 4 : Atomic Structure
Chapter: 7th Science : Term 1 Unit 4 : Atomic Structure
Atomic number and Mass number
Atomic number and Mass number
If
all elements are made up of same type of electrons, protons and neutrons how
does a carbon atom differ from a iron atom? Further investigations led to the discovery
that the number of the protons inside the nucleus of an atom determines what
element it is. For Example if the nucleus has only one proton, then all such
atoms are hydrogen atom. If there are eight protons then that atom is oxygen.
Is the structure of the atom the same as the structure of
the solar system? Yes ! It is similar to the solar system. It has a core center
called nucleus and it has paths called orbits around the nucleus.
Atomic number (z)
The
number of electrons or protons in anatomis called the atomic number of that
atom .It is represented by the letter Z. If we know the atomic number of an atom,
we know the number of electrons or protons in it.
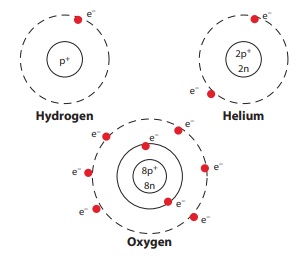
Look at the figures. The hydrogen
nucleus has one proton around which revolves one electron. It means that its
atomic number z=1.
In the helium atom there are two
protons and two electrons in orbit around the nucleus, so the atomic number of
helium is z=2.
Look at the atomic structure of
oxygen shown in the figure. What is its atomic number?
Try yourself
If the atomic number of carbon is Z=6, what is the number
of the electrons revolving in its atom
Mass number (A) or
Atomic mass :
We have seen that the mass of an
atom is concentrated in its nucleus. From this, we can get the atomic mass
number. mass number (A) is equal to the sum of the number of protons(p) and
neutrons (n) in the nucleus.
Automic mass or mass number = Number
of Protons + Number of Neutrons
A = p+n
A lithium atom contains 3 Protons
and 4 neutrons . Its atomic mass number A = 3+4 = 7.
In a sodium atom, there are 11
Protons and 12 neutrons. Hence , its atomic mass number A=11+12=23.
Try yourself
1. Why are atomic numbers and mass numbers are always whole
numbers ?
2. A sulphur atom contains 16 Protons and 16 neutrons .
Give its atomic number and atomic mass number.
When writing the symbol of an
element, its atomic number and atomic mass number are also written. For
example, the symbols of hydrogen, carbon and oxygen are written as 1H1,
6C12, 8O16 respectively.
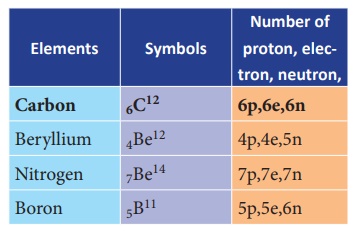
All the elements in the periodic
table have the following combination of protons, electrons and neutrons:
Isotopes: Atoms of the same element can have
different number of neutrons. Such atoms will have same atomic number but
different mass numbers. These atoms are called isotopes. For example Hydrogen
has three isotopes ---Hydrogen (1H1), Deuterium (1H2),
Tritium (1H3).
Isobars: Atoms that have the same mass number but different atomic numbers. for example Calcium – 40 and Argon – 40
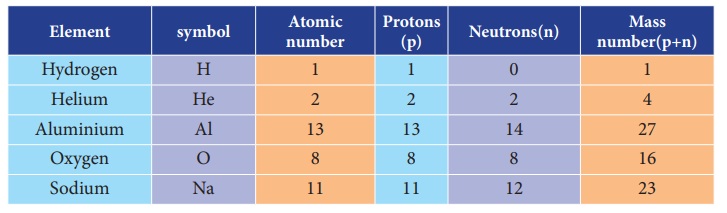
Elements and their symbols with
their atomic number and mass number.
ACTIVITY 3
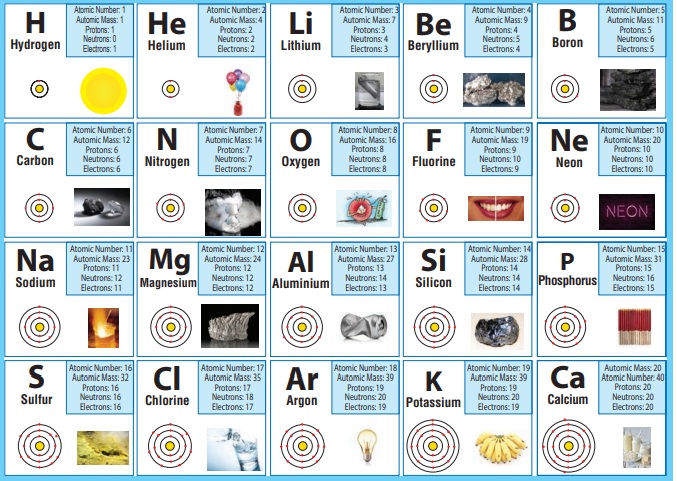
Observe the table
given above and answer the following questions.
1. I am used for
breathing, without me you cannot live. Do you know me? Write my name and symbol Oxygen, O.
2. It is used in filling the balloons. It is a gas, identity it. What is its mass number? Hydrogen. (A=l).
3. Name the element present in banana. What is my atomic number? Potassium (Z = 19)
4. I am found in crackers. How many protons do i have? Sulfur (p = 16)
5. I am the most valuable element. Find who am I? Can you say my mass number? Carbon (A= 12)
Related Topics