Atomic Structure | Term 1 Unit 4 | 7th Science - Student Activities | 7th Science : Term 1 Unit 4 : Atomic Structure
Chapter: 7th Science : Term 1 Unit 4 : Atomic Structure
Student Activities
ACTIVITY 1
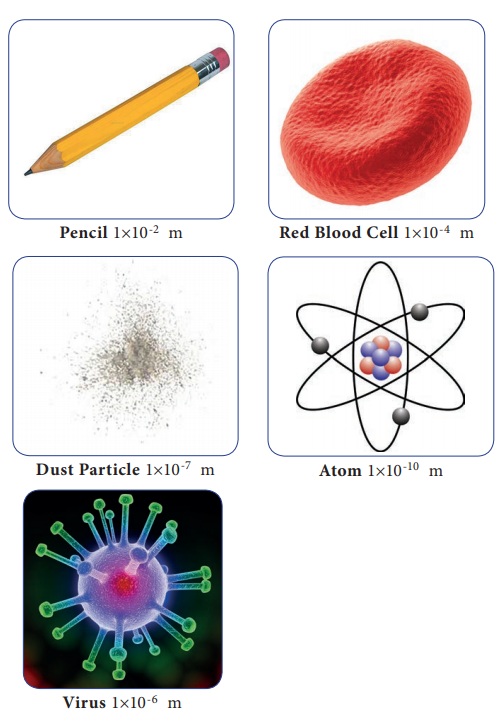
Some known objects are shown, also the broken particles of the objects are shown.
1. Name the articles or objects you see here? Also try to write What each of it made of?

1. Hammu (Iron)
2. Bangles (gold)
3. Tap (nickel)
4. Versal (copper)
Now you could imagine how small an atom would be.
ACTIVITY 2
Let us learn the characteristics of the subatomic particles through the following activity. Label the parts in the given diagram and answer the following.
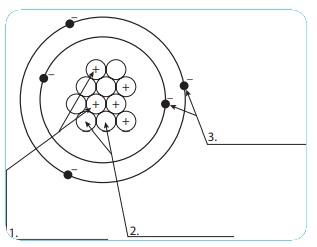
1. The positively charged particleis proton.
2. The negatively charged particleis electron.
3. Electron is neutral.
Try yourself
If the atomic number of carbon is Z=6, what is the number of the electrons revolving in its atom
Try yourself
1. Why are atomic numbers and mass numbers are always whole numbers ?
2. A sulphur atom contains 16 Protons and 16 neutrons . Give its atomic number and atomic mass number.
ACTIVITY 3
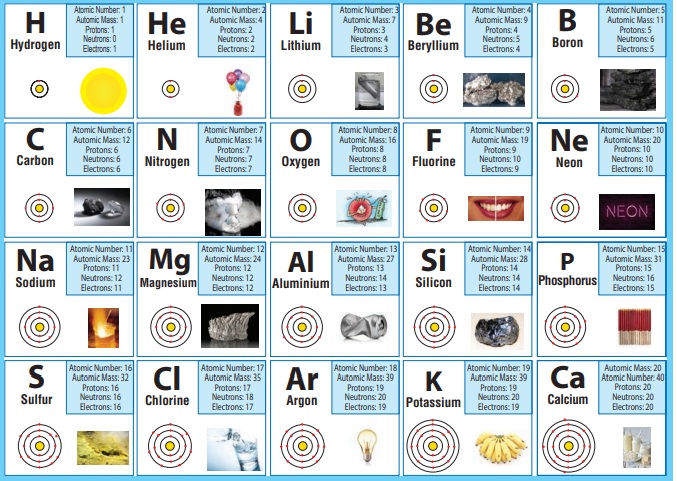
Observe the table given above and answer the following questions.
1. I am used for breathing, without me you cannot live. Do you know me? Write my name and symbol Oxygen, O.
2. It is used in filling the balloons. It is a gas, identity it. What is its mass number? Hydrogen. (A=l).
3. Name the element present in banana. What is my atomic number? Potassium (Z = 19)
4. I am found in crackers. How many protons do i have? Sulfur (p = 16)
5. I am the most valuable element. Find who am I? Can you say my mass number? Carbon (A= 12)
Related Topics