Atomic Structure | Term 1 Unit 4 | 7th Science - Evolution of idea of an atom | 7th Science : Term 1 Unit 4 : Atomic Structure
Chapter: 7th Science : Term 1 Unit 4 : Atomic Structure
Evolution of idea of an atom
Evolution of idea of
an atom
Many scientists have studied the
structure of the atom and advanced their theories about it. The theories proposed by
Dalton,Thomson and Rutherford are given below.
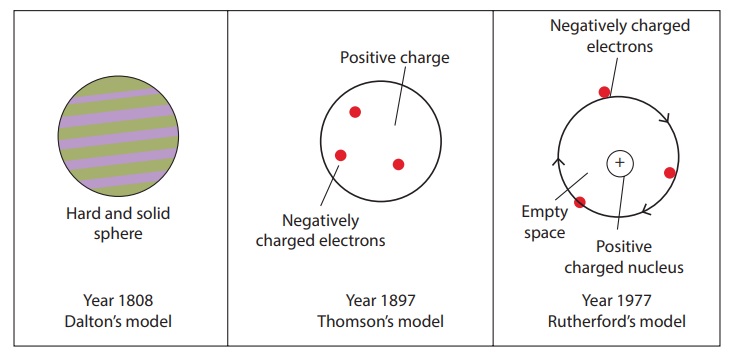
Dalton’s atomic theory
John Dalton proposed the atomic
theory in the year 1808. He proposed that matter consists of very small particles
which he named atoms. An atom is smallest indivisible particle, it is
speherical in shape. His theory does not propose anything about the positive
and negative charges of an atom.

Hence , it was not able to explain
many of the properties of substances.
Nanometer is the smallest unit used to measure small
lengths. One metre is equal to 1×10-9 nm or one nanometer is equal
to 1×10-9 m
Thomson’s theory
In 1897 J.J Thomoson proposed a
different theory. He compared an atom to a watermelon.

His theory proposed that the atom
has positively charged part like the red part of the watermelon andin it are
embedded , like the seeds, negatively charged particles which he called
electrons. According to this theory as the positive and negative charges are
equal, the atom as a whole does not have any resultant charge.
Thomson’s greatest contribution was
to prove by experimentation the existence of the negatively charged particles
or electrons in an atom. For this discovery, he was awarded the Nobel Prize in
1906. Although this theory explained why an atom is neutral , it was an
incomplete theory in other ways.
Rutherford’s theory
There were short comming in
Thomson's theory, Earnest Rutherford gave a better understanding. Earnest Rutherford
conducted an experiment. He Rutherford bombarded a very thinlayer of gold with
positively charged alpha rays. He found that most of these rays which travel at
a great velocity passed through th gold sheet without encountering any
obstacles. A few are, however, turned back from the sheet.

Rutherford considered this
remarkable and miraculous as if a bullet had turned back after colliding with
tissue paper.
Based on this experiment, Rutherford
proposed his famous theory. In his opinion, – 1. The fact that most alpha
particles pass through the gold sheet means that the atom consists mainly of
empty space. 2. The part from which the positively charged particles are turned
back is positively charged but very small in size as compared to the empty
space.
From these inferences, Rutherford
presented his theory of the structure of atoms. For this theory, he was awarded
the Nobel prize for chemistry.
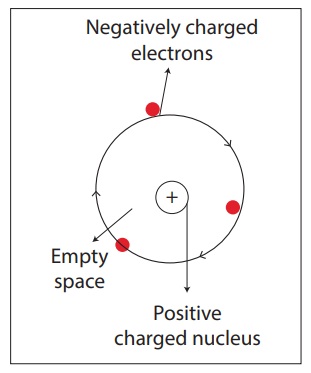
Rutherford’s theory
proposes that
1. The nucleus at the centre of the
atom has the positive charge. Most of the mass of the atom is concentrated in
the nucleus.
2. The negatively charged electrons
revolve around the nucleus in specific orbits.
3. In comparison with the size of
the atom, the nucleus is very very small
You have around 7 billion atoms in your body, yet you replace
about 98% of them every year!
Stages of discovery of the constituents of an atom
Evolution of the atomic
structure from the 5 elements
Related Topics