Chapter: Medical Surgical Nursing: Management of Patients With HIV Infection and AIDS
Treatment of HIV Infection
Treatment
of HIV Infection
Protocols of how to treat HIV disease change
relatively often. Yearly a team of physicians from throughout the United States
evaluates the latest evidence and makes recommendations that are widely
disseminated, and monthly a subgroup evaluates available evidence (Panel on
Clinical Practices for Treatment of HIV In-fection [Panel], 2002). Treatment
decisions for an individual pa-tient are based on three factors: HIV RNA (viral
load); CD4 T-cell count; and the clinical condition of the patient (Panel,
2000). Treatment should be offered to all patients with the pri-mary infection
(acute HIV syndrome, as previously described). In general, treatment should be
offered to individuals with a T-cell count of less than 350 or plasma HIV RNA
levels exceeding 55,000 copies/mL (RT-PCR assay) (Panel, 2002).
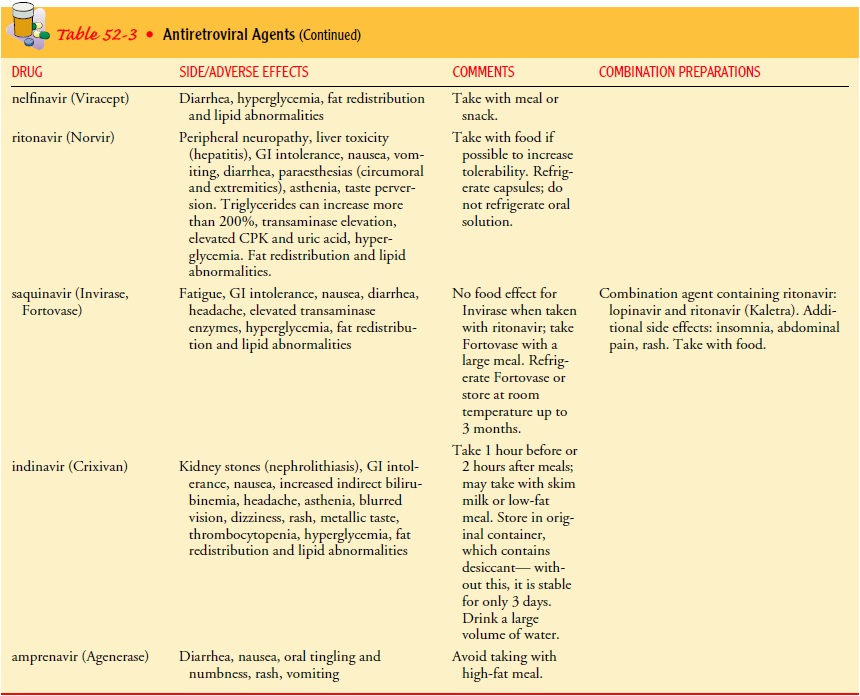
The increasing number of antiretroviral
agents (Table 52-3) and the rapid evolution of new information have introduced
ex-traordinary complexity into the treatment of HIV-infected per-sons (Panel,
2000). Adherence rates among persons living with HIV and AIDS are no different
from those of patients with other chronic diseases (Williams, 2001).
Antiretroviral regimens are complex, have major side effects, pose difficulties
with regard to adherence, and carry serious potential consequences from the
de-velopment of viral resistance due to lack of adherence to the drug regimen
or suboptimal levels of antiretroviral agents (Panel, 2000). The goals of
treatment are maximal and durable suppres-sion of viral load, restoration
and/or preservation of immunologic function, improved quality of life, and
reduction of HIV-related morbidity and mortality. The Panel’s guidelines
recommend viral load testing at diagnosis and every 3 to 4 months thereafter in
the untreated person; T-cell counts should be measured at diagnosis and
generally every 3 to 6 months thereafter.

It is
difficult to predict which patients will adhere to medica-tion regimens
(Holzemer, Corless, Nokes, et al., 1999). Perceived engagement with the health
care provider has been associated with greater adherence to HIV medication
regimens (Bakken et al., 2000). Individualized plans of care that take into
consideration housing and social support issues in addition to health indicators
are essential.
Results of therapy are evaluated with viral
load tests (Panel, 2000). Viral load levels should be measured immediately
prior to and again at 2 to 8 weeks after initiation of antiretroviral therapy,
since in most patients adherence to a regimen of potent anti-retroviral agents
should result in a large decrease in the viral load by 2 to 8 weeks. The viral
load should continue to decline over the following weeks and in most
individuals will drop below de-tectable levels (currently defined as less than
50 RNA copies/mL) by 16 to 20 weeks. The rate of viral load decline toward
unde-tectable levels is affected by the baseline T-cell count, the initial
viral load, the potency of the medication, adherence of the pa-tient to the
medication regimen, prior exposure to antiretroviral agents, and the presence
of any OIs (Panel, 2000). The confirmed absence of a viral load response should
prompt the health care team to re-evaluate the regimen.
All approved anti-HIV drugs attempt to block
viral replica-tion within cells by inhibiting either reverse transcriptase or
the HIV protease (Bartlett & Moore, 1998). A treatment duration of 5 to 7
years of continuous therapy is difficult because of the com-plexity, toxicity,
and cost of the current drug regimens, especially when the concept of
maintenance therapy with a simplified regimen does not seem viable (Ho, 1998).
Medication side effects can make life difficult and are one of the main reasons
people miss doses of the medications or stop taking them completely (Horn &
Pieribone, 1999).
All medications have toxic side effects. The
nurse can obtain Web-based information to remain current about medications used
to treat HIV/AIDS. The NIH (National Institutes of Health) maintains an AIDS
drug line Website. Increasing numbers of patients with HIV infection receiving
medications are presenting with metabolic complica-tions such as increases in
cholesterol and triglyceride levels, hyper-glycemia, and altered body habitus
(NIAID, 2001). Toxicity to cell mitochondria may be involved in many of the
side effects of HIV medications, including peripheral neuropathy, myopathy and
cardiomyopathy, lactic acidosis and hepatic steatosis (fatty degeneration of
liver), pancreatitis, osteopenia and osteoporosis, and bone marrow suppression.
Fat redistribution (lipodystrophy syndrome, also known as pseudo-Cushing’s
syndrome [Panel, 2001]) is one of the most frequent systemic side effects. Many
people who have lipodystrophy experience an increase in fat loss in the legs,
arms, and face and/or a buildup of fat around the ab-domen and at the base of
the neck. Patients may also experience an increase in breast size. These
changes in body image can be very disturbing to persons living with HIV/AIDS
and have been reported to occur in 6% to 80% of patients receiving HAART (see
following discussion). Hepatotoxicity associated with certain protease
inhibitors may limit the use of these agents, especially in patients with
underlying liver dysfunction (Panel, 2000).
Combination therapy is defined as a regimen
containing any combination of two antiretroviral agents; HAART is defined as a
regimen consisting of two nucleoside reverse transcriptase in-hibitors plus a protease inhibitor or a non-nucleoside
reverse transcriptase inhibitor, or two protease inhibitors and one other
antiretroviral agent (Agins, 2000). As new medications are devel-oped, the
number of combinations continues to increase. Safety and efficacy data on many
of the combination therapies are lim-ited. Use of three- and four-drug combination
regimens has be-come more widespread, starting earlier in the course of
infection, with careful monitoring by viral load measures. In some patients
receiving three-drug regimens, viral levels are so low that they are no longer
detectable. Future therapy may be individualized based on the viral strain and
resistance to antiretroviral drugs. Initially, HAART consisting of a
triple-drug regimen (a protease inhibitor and two non-nucleoside reverse
transcriptase inhibitors) is rec-ommended. Drawbacks of HAART are the inability
of some patients to adhere to the regimen, the need to take multiple
medications on different dosing schedules, and the risk for drug interactions.
The duration of therapy needed to control acute HIV infection is unknown, but therapy
may continue for several years or for life. Combination therapy with different
types of fusion and entry inhibitors (such as T-20) may be synergistic against
HIV. These agents fall into a new category of HIV med-ications called fusion
inhibitors and target the GP120 during the initial stage of the HIV life cycle,
which is cell fusion (Saag, 2001).
Related Topics