Chapter: Medical Surgical Nursing: Management of Patients With HIV Infection and AIDS
HIV Infection and AIDS: Assessment and Diagnostic Findings
Assessment
and Diagnostic Findings
During the first stage of HIV infection, the
patient may be asymp-tomatic or may exhibit various signs and symptoms. The
patient history should alert the health care provider about the need for HIV
screening based on the patient’s sexual practices, injection drug use, and
receipt of blood transfusions. Additionally, exposure to body fluids containing
infected blood while providing care to others with HIV infection, such as
through needlesticks, should alert health care providers to possible HIV
infection. Patients who are in later stages of HIV infection may have a variety
of symp-toms related to their immunosuppressed state.
Several
screening tests are used to diagnose HIV infection.
Others
are used to assess the stage and severity of the infection.
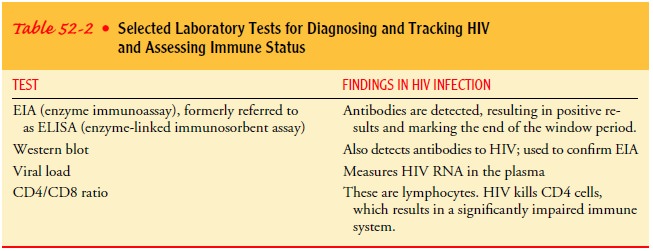
Table
52-2 identifies common blood tests.
HIV ANTIBODY TESTS
In 1985, the FDA licensed an HIV-1 antibody
assay, which is used to screen all blood and plasma donations. When an
indi-vidual is infected with HIV, the immune system responds by pro-ducing
antibodies against the virus, usually within 3 to 12 weeks after infection.
This delay in the production of antibody helps to explain why a person may be
infected but not test antibody-positive during primary infection. The ability
to document HIV antibodies in the blood has permitted screening of blood
products and facilitated identification of individuals with HIV infection.
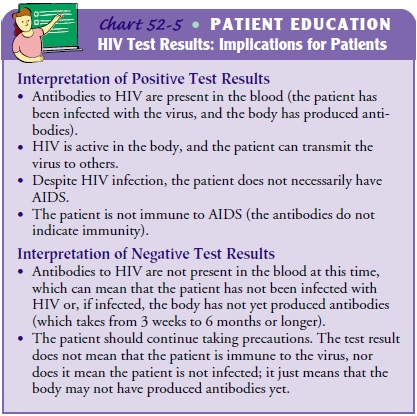
Before an HIV antibody test is performed, the meaning of the test and possible
test results are explained, and informed consent for the test is obtained from
the patient. When results of the HIV antibody testing are received, they are
carefully explained to the patient in private (Chart 52-5). All test results are
kept confiden-tial. Education and counseling about the test results and disease
transmission are essential.
Blood samples are tested with two different blood tests to determine the presence of antibodies to HIV. The EIA (enzymeimmunoassay), formerly referred to as the ELISA (enzyme-linked immunosorbent assay) test, identifies antibodies directedspecifically against HIV. The Western blot assay is used to confirm seropositivity when the EIA is positive. People whose blood contains antibodies for HIV are seropositive. Saliva can also be tested using the EIA antibody test.
The
FDA approved a rapid HIV antibody screening test in November 2002. Using less
than a drop of blood, this new test can quickly (approximately 20 minutes) and reliably
(99.6% ac-curacy) detect antibodies to HIV-1. It is anticipated that the newly
approved HIV test, the OraQuick Rapid HIV-1 Antibody Test, will allow more
rapid notification of individuals about their HIV status so that they can
obtain care early in the course of HIV infection and take measures to help
prevent the spread.
Patients’ psychological responses to
seropositive test results may include feelings of panic, depression, and
hopelessness. The social and interpersonal consequences of a positive test
result can be devastating. Patients may lose their sexual partners and their
health insurance because of disclosure; they may experience dis-crimination in
employment and housing as well as social os-tracism. For these reasons and
others, patients who test positive may need ongoing counseling as well as
referrals for social, financial, medical, and psychological support services.
Patients whose test results are seronegative may develop a false sense of
se-curity, possibly resulting in continued high-risk behaviors or feel-ings
that they are immune to the virus. They may need ongoing counseling to help
them modify high-risk behaviors and to return for repeated testing. Other
patients may experience anxiety re-garding the uncertainty of their status.
Home-based testing for HIV antibodies using a
small amount of blood was first proposed in 1985 but not approved by the FDA
until 1995. Although home testing kits are commercially avail-able, they do
raise concerns because of the lack of counseling and the possibility of
inaccurate results, including both false-positive and false-negative results
(Lewis, 2001).
VIRAL LOAD TESTS
Target amplification methods quantify HIV RNA
or DNA lev-els in the plasma and have replaced p24 antigen capture assays.
Target amplification methods include reverse transcriptase poly-merase chain reaction (RT-PCR) or nucleic acid sequence-based
amplification (NASBA). A widely used viral
load test measures plasma HIV RNA levels. Currently, these tests are used
to track viral load and response to treatment for HIV infection. RT-PCR is also
used to detect HIV in high-risk seronegative people before the development of
antibodies, to confirm a positive EIA, and to screen neonates. HIV culture or
quantitative plasma culture and plasma viremia are additional tests that
measure viral burden, but they are used infrequently. Viral load is a better
predictor of the risk of HIV disease progression than the CD4count. The lower the
viral load, the longer the time to AIDS diagnosis and the longer the survival
time.
Related Topics