Chapter: The Massage Connection ANATOMY AND PHYSIOLOGY : Respiratory System
Transport of Gases - Respiratory System
TRANSPORT OF GASES
As already mentioned, the solubility coefficient of oxygen is low. Given the difference in partial pressure of oxygen and its solubility coefficient, only about 0.3 mL (0.02 in3) of oxygen can be transported dissolved in 100 mL (6.1 in3) of plasma. This is grossly insuffi-cient for the tissue. With carbon dioxide, much more is produced in the tissue than can be carried dissolved in plasma. Hence, a more efficient mechanism is needed to transport the gases. Hemoglobin, present in red blood cells, with its great affinity for oxygen, en-ables blood to carry a larger quantity of the gases.
Transport of Oxygen
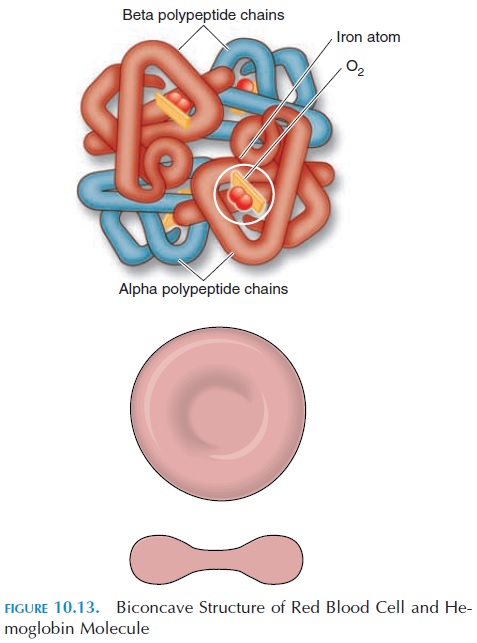
The primary function of a red blood cell is to trans-port oxygen and carbon dioxide, and its structure is modified to suit this function. As red blood cells ma-ture, all cellular components not directly related to function are lost. For example, mature red cells do not have a nucleus. Ninety-five percent of intracellu-lar protein in these cells is hemoglobin. The cell is small, about 7.4 µ, and shaped like a biconcave disk, with a narrow central part. This structure helps the cell squeeze through the tiny capillaries without rup-turing (see Figure 10.13).
Unique Properties of Hemoglobin
The hemoglobin molecule is made up of four globular protein chains, two alpha and two beta chains (Fig-ure 10.13). Each chain contains a pigment complex known as heme, which has an attached iron ion. The iron component of the hemoglobin molecule com-bines with oxygen to form oxyhemoglobin. The link-age of iron to oxygen is weak and can be easily broken in accordance with the oxygen concentration in the surrounding tissue. Each hemoglobin molecule is ca-pable of combining with four molecules of oxygen. There are approximately 280 million hemoglobin mol-ecules in each red blood cell and about 98.5% of the oxygen carried in the blood is bound to hemoglobin.
Hb + O2↔ Hbo2
Reduced hemoglobin + Oxygen ↔ Oxyhemoglobin
The amount of oxygen bound to hemoglobin de-pends on the oxygen level of its surroundings. If the oxygen level is low, hemoglobin releases the oxygen and carbon dioxide binds to the globin chains. In the capillaries of the lungs, where oxygen levels in the alveolar air are high, carbon dioxide is released and oxygen binds to the hemoglobin. The activity of cells can be maintained only if the hemoglobin levels are within normal range.
Factors Affecting the Binding Property of Hemoglobin
The affinity of hemoglobin for oxygen is unique (see Figure 10.14), which shows the affinity of hemoglo-bin for oxygen at different partial pressures of oxy-gen. The affinity varies with the partial pressure of oxygen around it, changes in temperature, and pH. As the partial pressure of oxygen increases around hemoglobin, more oxygen is attached to hemoglobin (i.e., all four sites for oxygen in the heme component of hemoglobin molecule are occupied). When all four sites are attached to oxygen, the hemoglobin is con-sidered fully saturated with oxygen. If two sites are occupied, hemoglobin is considered partially satu-rated; in this case, 50% saturated. In the alveoli, the partial pressure of oxygen is high and hemoglobin is almost fully saturated. Note that the hemoglobin is more than 90% saturated even when the partial pres-sure is as low as 60 mm Hg (1.16 psi). This is advan-tageous because hemoglobin can combine with large quantities of oxygen even when the partial pressure drops as low as 60 mm Hg (as in high altitudes or with some respiratory disorders).
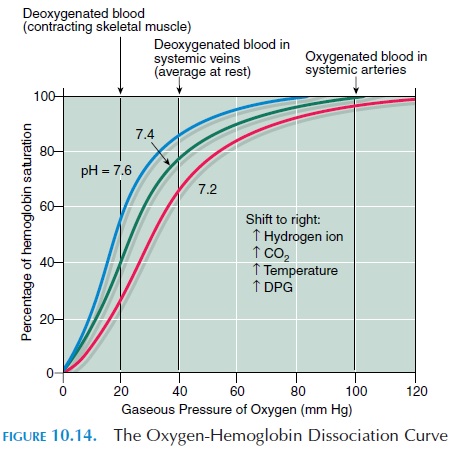
When the partial pressure of oxygen drops below 40 mm Hg (0.77 psi), the hemoglobin becomes less and less saturated when the partial pressure drops even a little. This characteristic is good because the partial pressure around tissue cells is less than 40 mm Hg. When oxygenated blood reaches the tissue cells, because of this characteristic, oxygen is rapidly and easily detached from hemoglobin. In active tis-sue, the partial pressure drops even more and even more oxygen is unloaded from hemoglobin.
When the pH drops (i.e., becomes more acidic or the temperature increases slightly), the oxygen-hemoglobin dissociation curve is shifted to the right (Figure 10.14). Also, the accumulation of compound 2,3 diphosphoglycerate (2,3 DPG), which is formed plentifully during metabolism in the red blood cells, facilitates dissociation of oxygen from hemoglobin. This implies that hemoglobin gives up oxygen easily at a higher partial pressure of oxygen. In active tis-sue, the temperature increases slightly because of the increase in metabolism. The liberation of carbon dioxide and production of lactic acid make the envi-ronment more acidic. All of these factors—change in temperature, drop in pH, and accumulation of 2,3 DPG—alter the characteristic of hemoglobin, making it unload more oxygen even at a higher partial pres-sure of oxygen. In this way, the tissue increase oxygen availability according to their activity.
High Altitude and Hemoglobin Levels
At high altitudes, the partial pressure of oxygen in the atmosphere is low, lowering the availability of oxygen in the air. The body adapts to the low levels by in-creasing red blood cells and hemoglobin content. This way, even if the hemoglobin molecules do not get saturated with oxygen, there are more in number to combine with oxygen. The increase in hemoglobin content and red blood cells is initiated by the hor-mone erythropoietin , whose levels in-crease when oxygen levels drop.
Transport of Carbon Dioxide
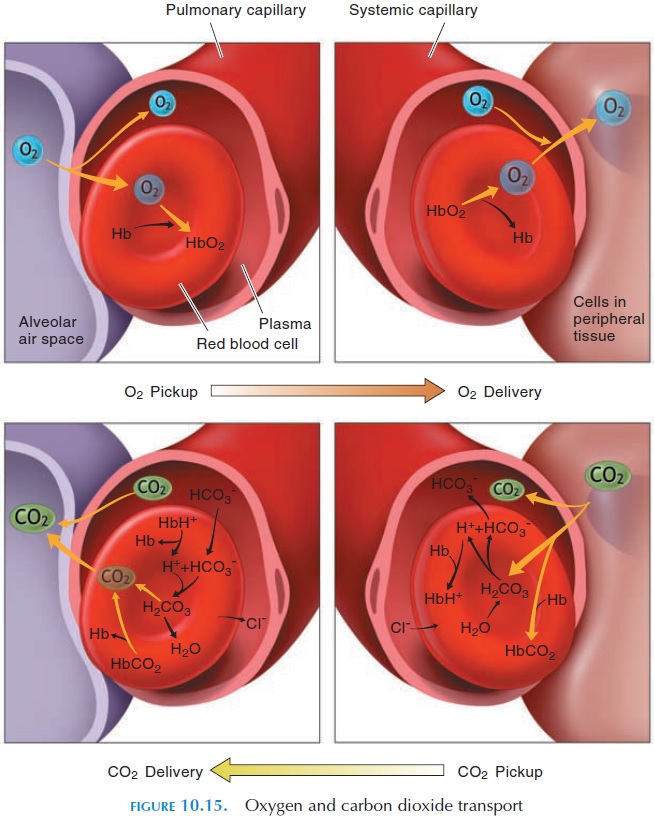
Carbon dioxide, a byproduct of aerobic metabolism, is transported in the blood in three ways that are re-versible (see Figure 10.15). Because it is about 20 times more soluble in blood than oxygen, about 7% of carbon dioxide dissolves in plasma. Twenty-three percent combine with hemoglobin to form car-baminohemoglobin. The remaining 70% are con-verted to carbonic acid in the presence of the enzyme carbonic anhydrase found inside red blood cells. The carbonic acid dissociates easily and rapidly into hy-drogen ions and bicarbonate ions.
CO2 H2O ↔ H2CO3↔ H HCO3
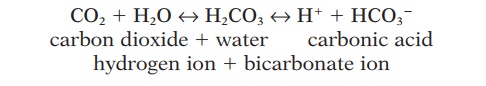
Most hydrogen ions formed in this reaction bind to hemoglobin molecules. Thus, hemoglobin mole-cules serve as buffers, preventing these ions from al-tering the pH of blood. The bicarbonate ions move out of the red blood cells into the plasma in exchange for chloride ions, which move into the cells from the plasma (chloride shift). Because deoxygenated he-moglobin forms carbamino compounds more easily and has a greater affinity for hydrogen ions than oxy-genated hemoglobin, transport of carbon dioxide is facilitated in venous blood.
Related Topics