Chapter: The Massage Connection ANATOMY AND PHYSIOLOGY : Respiratory System
Gas Exchange - Respiratory System
Gas Exchange
Gas movement between the alveoli and blood is by passive diffusion. The rate of gas exchange can be ex-plained using simple physical laws. Dalton’s law states that each gas in a mixture of gases exerts its own pressure, behaving as if it is the only gas present. The pressure exerted by the gas is known as partialpressure (of that gas). The addition of the partialpressure of all the gases in the mixture will be equal to the total pressure exerted by the mixture of gases. In atmospheric air, the total pressure exerted at sea level is equal to 760 millimeters of Mercury (mm Hg) or 14.7 pounds per square inch (psi). This pressure can be obtained by the addition of the partial pres-sure of nitrogen (78.6%), oxygen (20.9%), carbon dioxide (0.04%), water (0.4%), and vapor and other gases (0.06%) in the atmosphere. The partial pressure of the gas can be easily determined by multiplying the percentage of the gas in the atmosphere by total atmospheric pressure. Atmospheric air has 20.9% oxygen. Then the partial pressure of oxygen in a re-gion where the atmospheric pressure is equal to 760 mm Hg is: 760 x 0.209 158.8 mm Hg (14.7 0.209 3.07 psi). At high altitudes, where the atmospheric pressure is lower, the partial pressure of oxygen would drop even though the percentage of oxygen is the same, directly affecting the rate of gas exchange. For example, if the atmospheric pressure is 740 mm Hg (14.31 psi), then the partial pressure of oxygen would be: 740 x 0.209 = 154.66 mm Hg (14.31 x 0.209 = 2.99 psi). That is why, before getting accli-matized, you feel out of breath when you ascend to high altitudes.
Gases move across a permeable membrane from an area of higher partial pressure to an area of lower partial pressure. The rate at which each gas moves from a mixture of gases is determined by the differ-ence in its partial pressure. The partial pressure of other gases in the mixture is not a factor. For example, the movement of oxygen in the lungs from the alveoli to the blood is determined by the difference in partial pressure of oxygen in the alveoli and the blood, not by the partial pressure of carbon dioxide or other gases.
Other than partial pressure, the quantity of gas moving across the membrane and dissolving in blood would be determined by how soluble the gas is in plasma. Henry’s law describes the behavior of gas. Henry’s law states that the quantity of gas that will dissolve in a liquid is proportional to the partial pres-sure of the gas and its solubility coefficient (the vol-ume of gas that dissolves in one unit volume of a liq-uid at a particular temperature). Carbon dioxide in the body has a higher solubility coefficient (24 times more) than oxygen. Therefore, more carbon dioxide is carried dissolved in plasma. Henry’s law explains why we have little nitrogen dissolved in plasma. Al-though the air has a high partial pressure of nitrogen (79%), little dissolves in plasma because of the low solubility coefficient of nitrogen.
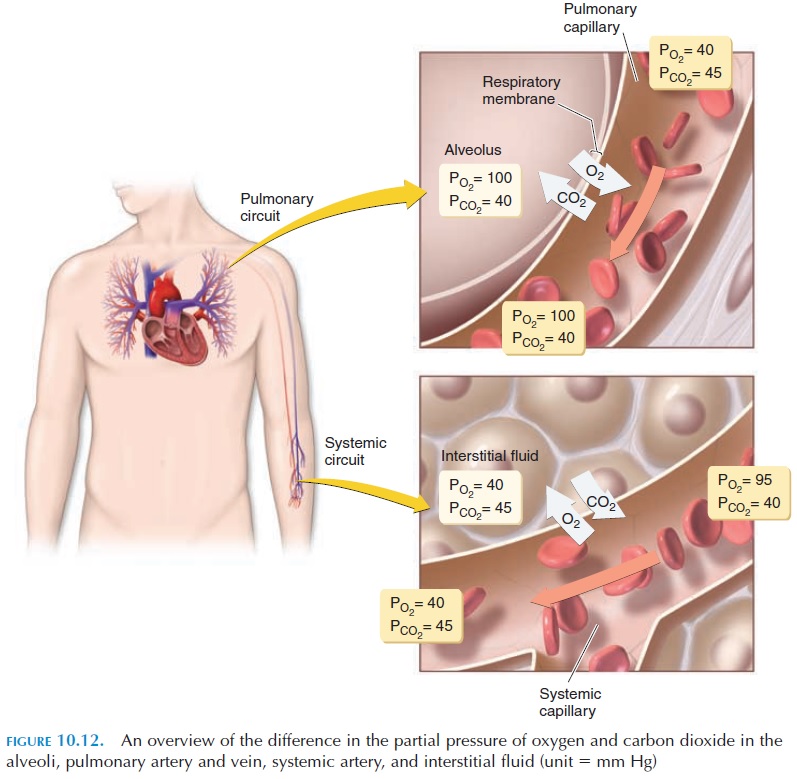
Figure 10.12 is an overview of the difference in the partial pressure of oxygen and carbon dioxide in the alveoli, pulmonary artery and vein and in the sys-temic artery, vein, and the interstitial fluid.
In addition to the partial pressure and solubility coefficient of carbon dioxide and oxygen, the rate of exchange would be affected by the thickness of the respiratory membrane and the surface area available for exchange. For example, when there is pulmonary edema, fluid in the respiratory membrane increases the distance through which diffusion must take place, with consequent reduction in rate of gas ex-change. If a lobe of the lung is collapsed, the surface area for exchange is reduced and less oxygen diffuses.
As the deoxygenated blood in the pulmonary artery passes around the alveoli, exchange of gases occurs quickly, with oxygen moving into the blood from the alveolar air and carbon dioxide from the blood into the air. Although the red blood cells in the blood stay in the capillaries for less then a second (in an exercising individual, about 0.3 seconds), the time is sufficient for exchange to occur.
Related Topics