Chapter: Basic & Clinical Pharmacology : Thyroid &Antithyroid Drugs
Thyroid Physiology
THYROID PHYSIOLOGY
The
normal thyroid gland secretes sufficient amounts of the thyroid hormones—triiodothyronine (T3) and tetraiodothyronine(T4, thyroxine)—to
normalize growth and development, bodytemperature, and energy levels. These
hormones contain 59% and 65% (respectively) of iodine as an essential part of
the molecule. Calcitonin, the second type of thyroid hormone, is important in
the regulation of calcium metabolism.
Iodide Metabolism
The recommended daily
adult iodide (I–)∗ intake is 150 mcg (200 mcg during
pregnancy).Iodide, ingested from food, water, or medication, is rapidly
absorbed and enters an extracellular fluid pool. The thyroid gland removes
about 75 mcg a day from this pool for hormone synthesis, and the balance is
excreted in the urine. If iodide intake is increased, the fractional iodine
uptake by the thyroid is diminished.
Biosynthesis of Thyroid Hormones
Once taken up by the
thyroid gland, iodide undergoes a series of enzymatic reactions that
incorporate it into active thyroid hormone (Figure 38–1). The first step is the
transport of iodide into the thy-roid gland by an intrinsic follicle cell
basement membrane protein called the sodium/iodide symporter (NIS). This can be
inhibited by such anions as thiocyanate (SCN–), pertechnetate (TcO4–), and perchlorate
(ClO4–). At the apical cell
membrane a second I– trans-port enzyme called pendrin controls the flow of iodide
across the membrane. Pendrin is also found in the cochlea of the inner ear. If
pendrin is deficient or absent, a hereditary syndrome of goiter and
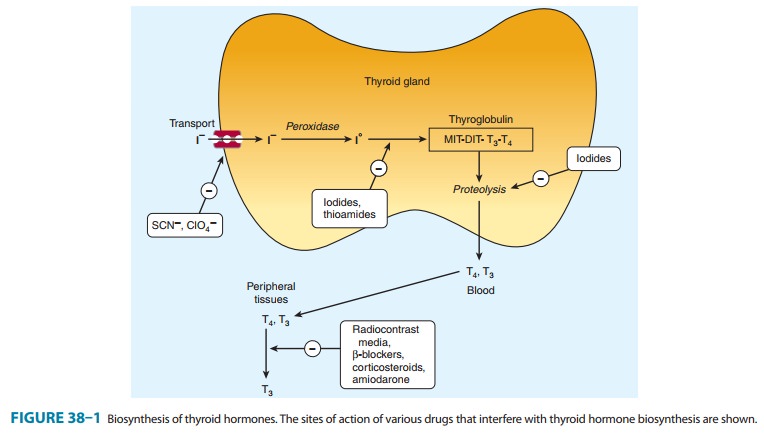
At the apical cell mem-brane, iodide is
oxidized by thyroidal peroxidase to iodine, in which form it rapidly iodinates
tyrosine residues within the thyroglobulin molecule to form monoiodotyrosine (MIT) and diiodotyrosine(DIT). This process is
called iodide organification. Thyroidal
per-oxidase is transiently blocked by high levels of intrathyroidal iodide and
blocked more persistently by thioamide drugs.
Two molecules of DIT
combine within the thyroglobulin mol-ecule to form L-thyroxine (T4). One molecule of MIT and one molecule of DIT combine to form T3. In addition to
thyroglobulin,other proteins within the gland may be iodinated, but these
iodo-proteins do not have hormonal activity. Thyroxine, T3, MIT, and DIT are
released from thyroglobulin by exocytosis and proteolysis of thyroglobulin at
the apical colloid border. The MIT and DIT are then deiodinated within the
gland, and the iodine is reutilized. This process of proteolysis is also
blocked by high levels of intrathyroidal iodide. The ratio of T4 to T3 within thyroglobulin
is approximately 5:1, so that most of the hormone released is thy-roxine. Most
of the T3 circulating in the
blood is derived from peripheral metabolism of thyroxine (, Figure 38–2).
Transport of Thyroid Hormones
T4
and T3 in plasma are reversibly bound to
protein, primarily thyroxine-binding globulin (TBG). Only about 0.04% of total
T4 and 0.4% of T3
exist in the free form. Many physiologic and pathologic states and drugs affect
T4, T3,
and thyroid transport. However, the actual levels of free hormone generally
remain nor-mal, reflecting feedback control.
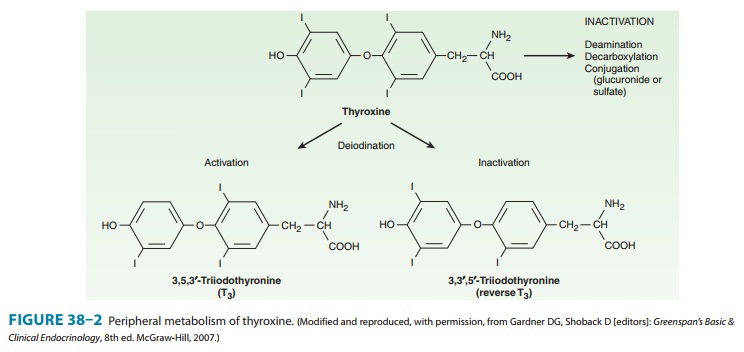
Peripheral Metabolism of Thyroid Hormones
The primary pathway
for the peripheral metabolism of thyroxine is deiodination. Deiodination of T4 may occur by
monodeiodina-tion of the outer ring, producing 3,5,3’-triiodothyronine (T3), which is three to
four times more potent than T4. Alternatively, deiodina-tion may occur in the inner ring,
producing 3,3’,5’-triiodothyronine (reverse T3, or rT3), which is metabolically inactive (Figure
38–2). Drugs such as amiodarone, iodinated contrast media, β blockers, and
corticosteroids, and severe illness or starvation inhibit the 5’-deiodinase
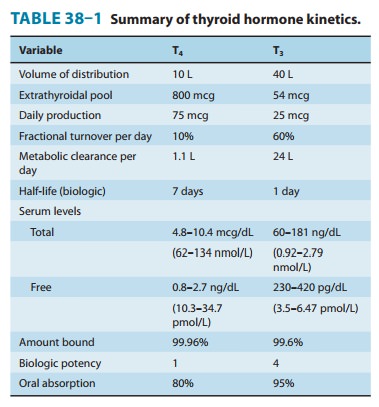
necessary for the conversion of T4 to T3, resulting in low T3 and high rT3 levels in the serum. The pharmacokinetics of
thyroid hormones are listed in Table 38–1. The low serum levels of T3 and rT3 in normal individuals
are due to the high metabolic clearances of these two compounds.
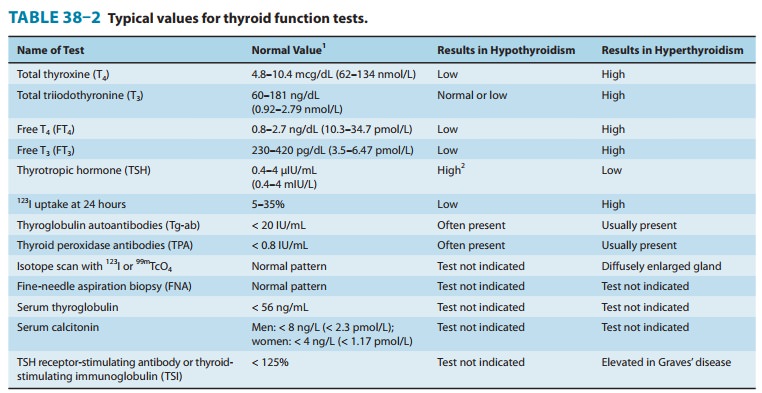
Evaluation of Thyroid Function
The tests used to
evaluate thyroid function are listed in Table 38–2.
A. Thyroid-Pituitary Relationships
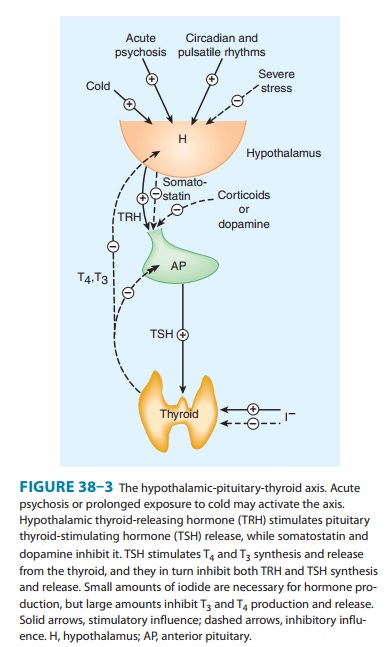
Briefly, hypothalamic cells secrete thyrotropin-releasing hormone (TRH) (Figure 38–3). TRH is secreted into capillaries of the pituitary portal venous system, and in the pituitary gland, TRH stimulates the synthesis and release of thyrotropin (thyroid-stimulating hormone, TSH). TSH in turn stimulates an adenylyl cyclase–mediated mechanism in the thyroid cell to increase the synthesis and release of T4 and T3. These thyroid hormones act in a negative feedback fashion in the pituitary to block the action of TRH and in the hypothalamus to inhibit the synthesis and secretion of TRH. Other hormones or drugs may also affect the release of TRH or TSH.
B. Autoregulation of the Thyroid Gland
The
thyroid gland also regulates its uptake of iodide and thyroid hormone synthesis
by intrathyroidal mechanisms that are inde-pendent of TSH. These mechanisms are
primarily related to the level of iodine in the blood. Large doses of iodine
inhibit iodide organification (Wolff-Chaikoff block, see Figure 38–1). In
certain disease states (eg, Hashimoto’s thyroiditis), this can inhibit thyroid
hormone synthesis and result in hypothyroidism. Hyperthyroidism can result from
the loss of the Wolff-Chaikoff block in susceptible individuals (eg,
multinodular goiter).
C. Abnormal Thyroid Stimulators
In Graves’ disease ,
lymphocytes secrete a TSH recep-tor-stimulating antibody (TSH-R Ab [stim]),
also known asthyroid-stimulating immunoglobulin (TSI). This immunoglobulin
binds to the TSH receptor and stimulates the gland in the same fashion as TSH
itself. The duration of its effect, however, is much longer than that of TSH.
TSH receptors are also found in orbital fibrocytes, which may be stimulated by
high levels of TSH-R Ab [stim] and can cause ophthalmopathy.
Related Topics